DULERA Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Dulera, and what generic alternatives are available?
Dulera is a drug marketed by Organon Llc and is included in one NDA.
The generic ingredient in DULERA is formoterol fumarate; mometasone furoate. There are nineteen drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the formoterol fumarate; mometasone furoate profile page.
Summary for DULERA
| US Patents: | 0 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Clinical Trials: | 4 |
| Formulation / Manufacturing: | see details |
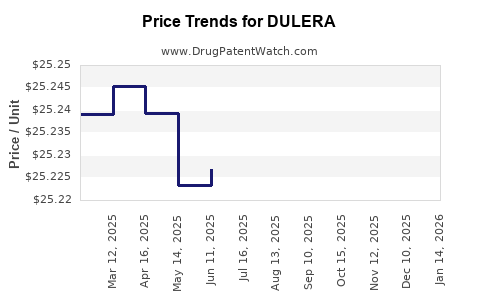
| Drug Prices: | Drug price information for DULERA |
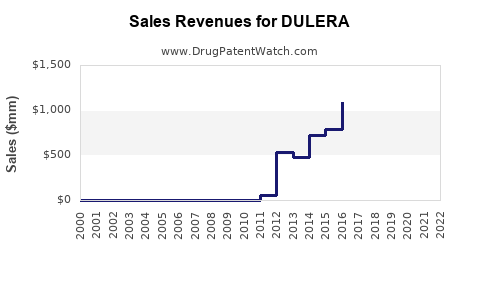
| Drug Sales Revenues: | Drug sales revenues for DULERA |
| What excipients (inactive ingredients) are in DULERA? | DULERA excipients list |
| DailyMed Link: | DULERA at DailyMed |
Recent Clinical Trials for DULERA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Sanofi | Phase 2 |
| Regeneron Pharmaceuticals | Phase 2 |
| West Penn Allegheny Health System | Phase 4 |
Pharmacology for DULERA
| Drug Class | Corticosteroid beta2-Adrenergic Agonist |
| Mechanism of Action | Adrenergic beta2-Agonists Corticosteroid Hormone Receptor Agonists |
Anatomical Therapeutic Chemical (ATC) Classes for DULERA
US Patents and Regulatory Information for DULERA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Organon Llc | DULERA | formoterol fumarate; mometasone furoate | AEROSOL, METERED;INHALATION | 022518-003 | Aug 12, 2019 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Organon Llc | DULERA | formoterol fumarate; mometasone furoate | AEROSOL, METERED;INHALATION | 022518-001 | Jun 22, 2010 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Organon Llc | DULERA | formoterol fumarate; mometasone furoate | AEROSOL, METERED;INHALATION | 022518-002 | Jun 22, 2010 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for DULERA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Organon Llc | DULERA | formoterol fumarate; mometasone furoate | AEROSOL, METERED;INHALATION | 022518-002 | Jun 22, 2010 | ⤷ Try a Trial | ⤷ Try a Trial |
| Organon Llc | DULERA | formoterol fumarate; mometasone furoate | AEROSOL, METERED;INHALATION | 022518-001 | Jun 22, 2010 | ⤷ Try a Trial | ⤷ Try a Trial |
| Organon Llc | DULERA | formoterol fumarate; mometasone furoate | AEROSOL, METERED;INHALATION | 022518-001 | Jun 22, 2010 | ⤷ Try a Trial | ⤷ Try a Trial |
| Organon Llc | DULERA | formoterol fumarate; mometasone furoate | AEROSOL, METERED;INHALATION | 022518-002 | Jun 22, 2010 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for DULERA
See the table below for patents covering DULERA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| South Africa | 9500637 | ⤷ Try a Trial | |
| Canada | 2362499 | COMBINAISONS DE FORMOTEROL ET DE FUROATE DE MOMETASONE DESTINEES A L'ASTHME (COMBINATIONS OF FORMOTEROL AND MOMETASONE FUROATE FOR ASTHMA) | ⤷ Try a Trial |
| European Patent Office | 1174139 | Utilisation du furoate de mometasone pour le traitement des affections pulmonaires et des voies respiratoires (Use of mometasone furoate for treating airway passage and lung diseases) | ⤷ Try a Trial |
| European Patent Office | 1174138 | Utilisation du furoate de mometasone pour le traitement des affections pulmonaires et des voies respiratoires (Use of mometasone furoate for treating airway passage and lung diseases) | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for DULERA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2435024 | 2021019 | Norway | ⤷ Try a Trial | PRODUCT NAME: KOMBINASJON AV FORMOTEROL; REG. NO/DATE: EU/1/20/1498 20201216 |
| 2435024 | PA2021511,C2435024 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: FORMOTEROLIO (ISKAITANT BET KOKIAS FARMACINIU POZIURIU PRIIMTINAS JO DRUSKAS, ESTERIUS, SOLVATUS ARBA ENATIOMERUS), GLIKOPIROLATO (ISKAITANT BET KOKIAS FARMACINIU POZIURIU PRIIMTINAS JO DRUSKAS, ESTERIUS, SOLVATUS ARBA ENANTIOMERUS) IR BUDEZONIDO (ISKAITANT BET KOKIAS FARMACINIU POZIURIU PRIIMTINAS JO DRUSKAS, ESTERIUS, SOLVATUS ARBA ENATIOMERUS) DERINYS; REGISTRATION NO/DATE: EU/1/20/1498 20201209 |
| 2435025 | 300995 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: COMBINATIE VAN GLYCOPYRRONIUM, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT OF ESTER, IN HET BIJZONDER GLYCOPYRRONIUYM BROMIDE, EN FORMOTEROL, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT OF ESTER, IN HET BIJZONDER FORMOTEROL FUMARAAT DIHYDRAAT; REGISTRATION NO/DATE: EU/ 1/18/1339 20181220 |
| 2435025 | PA2019014,C2435025 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: GLIKOPIRONIO BROMIDAS/FORMOTEROLIS; REGISTRATION NO/DATE: EU/1/18/1339 20181218 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


