Last updated: July 28, 2025
Introduction
DIVIGEL (estradiol hemihydrate) represents a transdermal estrogen hormone replacement therapy (HRT) predominantly prescribed to alleviate menopausal symptoms in women. As a topical gel formulation, DIVIGEL operates within the broader hormone therapy market, which is experiencing notable shifts driven by demographic trends, evolving regulatory landscapes, and technological innovations. This analysis explores the current market dynamics affecting DIVIGEL and forecasts its financial trajectory amid these changing environments.
Market Overview
Product Profile and Therapeutic Context
DIVIGEL delivers estradiol directly through the skin, circumventing hepatic first-pass metabolism and providing a steady delivery of hormone levels. Its primary indication—menopause management—addresses a significant global health concern, as women in the postmenopausal age bracket grow worldwide. The increasing prevalence of menopause-related symptoms amplifies demand for effective hormone therapy options, positioning DIVIGEL within a vital therapeutic niche.
Competitive Landscape
DIVIGEL competes primarily with other transdermal estrogen formulations (e.g., patches like Estraderm), oral HRT medications, and emerging non-hormonal alternatives. Major pharmaceutical firms with existing established brands and generic competitors influence market share distribution. The competitive intensity is compounded by patent expirations, regulatory approvals, and R&D investments by competitors exploring novel delivery systems or combination therapies.
Market Dynamics
1. Demographic and Epidemiological Drivers
The global aging population accelerates demand for menopause-related therapies. The World Health Organization estimates that by 2030, women aged 50+ will constitute over 20% of the global population, underpinning sustained growth in HRT markets. Additionally, heightened awareness regarding menopause management and increased healthcare access in developing economies boost potential user bases.
2. Regulatory and Policy Environment
Regulatory decisions significantly influence market dynamics. In recent years, increased scrutiny over hormone therapies, particularly post-Women’s Health Initiative (WHI) findings, led to stricter guidelines and periodic safety reevaluations. For DIVIGEL and similar products, such oversight affects approval pathways, labeling, and reimbursement policies, which can influence pricing strategies and market penetration.
3. Scientific and Technological Advances
Advancements in transdermal drug delivery systems—such as improved gels, patches, and implantable devices—enhance therapeutic efficacy and patient compliance. Minor modifications in formulation can extend patent life, deter generic competition, and influence revenue streams. Innovations also focus on minimizing side effects, a critical consideration in hormone therapy, impacting product acceptance.
4. Market Penetration and Patient Preferences
Patients increasingly favor non-invasive administration routes that offer convenience and consistent dosing. Transdermal gels like DIVIGEL provide these advantages over oral or injectable forms. Nonetheless, patient preference varies based on factors such as application convenience, perceived safety, and cost, affecting growth trajectories.
5. Reimbursement and Pricing Pressures
Healthcare payers wield considerable influence on market access, especially in major markets like the US, EU, and Japan. Cost-effectiveness evaluations, alongside negotiations and formulary placements, shape formulary inclusion and reimbursement levels for DIVIGEL, thereby impacting net revenues.
Financial Trajectory Analysis
1. Revenue Trends
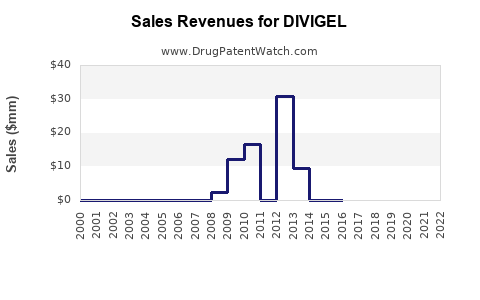
Historically, the global HRT market has experienced steady growth, driven by demographic factors and increased awareness. Although sales figures for DIVIGEL specifically are proprietary, market analysis indicates that transdermal estrogen formulations have gained a significant share—estimated at an annual growth rate (CAGR) of approximately 6-8% over recent years[1].
The product’s revenue outlook hinges on factors such as:
- Market Penetration: Expansion into emerging markets can offer high-growth opportunities, provided regulatory approvals are secured.
- Patent Status: Patent exclusivity enhances pricing power; patent expirations often lead to revenue erosion due to generic entry.
- Product Line Extensions: Development of combination gels (e.g., estradiol plus progesterone) or formulations with improved safety profiles can stimulate sales.
2. Cost Structure and Investment Needs
Research and development (R&D), manufacturing, regulatory compliance, and marketing constitute significant expenses. The highly regulated nature of hormone therapies necessitates continuous safety monitoring and clinical studies, impacting net profitability.
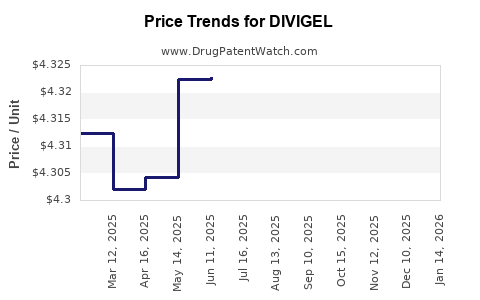
3. Competitive Pricing and Reimbursement Impact
Pricing strategies must balance profitability with affordability. In pricing-sensitive markets, reimbursement negotiations often compress margins. Larger players may leverage scale to offer more competitive pricing, intensifying downward pressure on DIVIGEL’s revenues.
4. Impact of Market Disruptors
Emerging therapies—such as selective estrogen receptor modulators (SERMs) and non-hormonal alternatives—pose competitive threats. Additionally, shifting regulatory stances on hormone safety could alter market accessibility and consumer confidence, affecting financial performance.
5. Sensitivity to External Factors
Economic downturns, healthcare policy reforms, and technological disruptions could impact traditional revenue streams. The COVID-19 pandemic demonstrated vulnerabilities in supply chains and market access but also accelerated adoption of telemedicine, influencing prescription dynamics.
Forecasting Future Financial Trajectory
Predictive models suggest that DIVIGEL's revenues could grow at a CAGR of approximately 5-7% over the next five years, aligned with overall hormone therapy market expansion and increasing acceptance of transdermal delivery systems[2]. The trajectory assumes stable regulatory environments, ongoing innovation, and strategic market entry in emerging economies.
However, potential challenges—such as patent cliffs, pricing pressures, and rapid technological shifts—could temper growth. Companies investing in next-generation formulations or combination therapies are likely to influence market share distribution significantly.
Key Factors Influencing Financial Outcomes
- Regulatory Approvals: Faster approvals in developing markets can unlock new revenue streams.
- Patent Strategy: Securing composition-of-matter patents for formulations prolongs exclusivity.
- Market Expansion: Geographic diversification reduces reliance on mature markets.
- Innovation Pipeline: R&D in safer, more convenient formulations enhances competitive advantage.
- Pricing and Reimbursement Strategies: Optimizing these mechanisms directly correlates with profitability.
Conclusion
DIVIGEL operates within a resilient yet competitive segment of the pharmaceutical industry, driven by demographic shifts and evolving patient preferences. Its future financial trajectory depends on effective regulatory navigation, innovation, strategic pricing, and expanding global access. While current trends favor modest growth, competitive pressures and regulatory challenges necessitate vigilant strategic planning to sustain profitability.
Key Takeaways
- The global aging demographic and increased awareness of menopause management support steady demand for DIVIGEL and similar therapies.
- Regulatory scrutiny and safety concerns continue to shape market access and product formulation strategies.
- Technological advancements and formulation innovations are critical to maintaining a competitive edge and extending patent protection.
- Market expansion into emerging economies presents significant growth opportunities, contingent upon regulatory approval and reimbursement frameworks.
- Strategic focus on innovation, patent protection, and cost management is vital to optimize the product’s financial performance amid competitive and regulatory pressures.
FAQs
1. What are the primary advantages of transdermal gels like DIVIGEL over oral hormone therapies?
Transdermal gels bypass the hepatic first-pass effect, providing more consistent hormone levels, reducing the risk of hepatic side effects, and improving adherence due to ease of use.
2. How does patent expiration impact DIVIGEL’s market position?
Patent expiration exposes the product to generic competition, typically leading to significant revenue declines unless the manufacturer develops new formulations or extension strategies.
3. What are major market risks facing DIVIGEL in the coming years?
Risks include regulatory policy shifts favoring non-hormonal treatments, safety concerns impacting public perception, pricing pressures, and the emergence of more advanced or alternative therapies.
4. How can companies extend the lifecycle of a product like DIVIGEL?
Through formulation improvements, combination therapies, securing new patents, expanding into untapped markets, and engaging in strategic collaborations.
5. What role do reimbursement policies play in DIVIGEL’s market success?
Reimbursement determines patient affordability and formulary placement, directly affecting sales volumes and revenue stability across different healthcare systems.
References
[1] MarketWatch. "Hormone Replacement Therapy Market Analysis." 2022.
[2] GlobalData. “Transdermal Drug Delivery Systems Market Forecast.” 2022.