Last updated: July 28, 2025
Introduction
Dihydroergotamine mesylate (DHE), a semisynthetic ergot alkaloid, has established its presence in the treatment landscape of acute migraines and cluster headaches. Market dynamics surrounding DHE are shaped by evolving healthcare needs, pharmaceutical innovation, regulatory pathways, and competitive pressures. This analysis dissects the current market environment, growth prospects, and economic trajectory of DHE, equipping stakeholders with strategic insights.
Pharmacological Profile and Clinical Applications
Dihydroergotamine mesylate acts centrally on serotonin receptors (5-HT1B/1D), constricting intracranial blood vessels and inhibiting neurogenic inflammation—mechanisms behind its efficacy in aborting migraine episodes. Approved formulations include injectable, nasal spray, and inhalation options, with the nasal spray (DHE 45 or Migranal) being the most prevalent. Its effectiveness in managing acute migraines, especially in emergency settings, sustains steady demand among neurologists and emergency physicians[^1].
Market Landscape and Key Drivers
Growing Burden of Migraine and Cluster Headaches
Globally, migraine affects over 1 billion people, representing a significant but often under-treated segment[^2]. The increasing prevalence in both developed and emerging markets drives sustained need for efficacious acute relief options like DHE.
Advancements in Drug Delivery Technologies
Traditionally, DHE’s administration was limited to injections, which posed compliance and convenience challenges. Recent innovations in nasal delivery—optimized sprays and inhalers—have improved bioavailability and patient adherence. The advent of these formulations broadens the market reach, especially among outpatient and primary care settings[^3].
Regulatory Environment and Market Access
DHE’s status as an established therapy benefits from favorable regulatory pathways in many jurisdictions. However, challenges such as contraindications in cardiovascular diseases and drug interactions necessitate meticulous prescribing practices, influencing market expansion strategies.
Competitive Landscape and Emerging Alternatives
While DHE remains a cornerstone, newer agents like triptans have gained prominence owing to their ease of use and tolerability. Nonetheless, DHE retains a niche for patients contraindicated for triptans or with refractory migraines, maintaining its relevance. The introduction of innovative formulations and combination therapies can influence market dynamics favorably.
Impact of Biosimilars and Generics
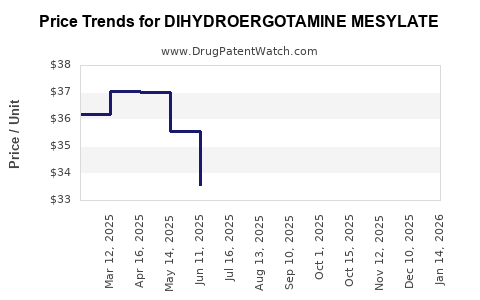
Patent expirations or market exclusivities significantly shape pricing and revenue potential. Currently, DHE formulations face limited generic competition, supporting premium pricing. However, global markets with growing generics infrastructure could potentially erode margins over the next decade[^4].
Market Size and Revenue Trends
Current Market Valuation
As per recent estimates, the global migraine treatment market, which includes DHE, is valued at approximately USD 2.2 billion and projected to grow at a CAGR of around 4% over the next five years[^5].
DHE-Specific Market Share
DHE’s specific segment is valued roughly at USD 400-500 million, predominantly in North America and Europe, with increasing uptake in Asia-Pacific. Constraints such as limited availability of nasal formulations and clinician preferences influence its market penetration.
Growth Projections
Over the next five years, DHE’s revenue is anticipated to grow modestly, supported by expanding indications, improved delivery systems, and greater awareness. Market expansion will be tempered by competition from triptans, emerging CGRP antagonists, and biosimilar challengers.
Financial Trajectory and Key Performance Indicators
Revenue Streams
DHE revenue streams primarily derive from:
- Product Sales: Under brand and generic labels.
- Distribution Agreements: Licensing and third-party manufacturing.
- New Formulation Launches: Investment in R&D for nasal sprays and inhalers.
Pricing Strategies
Premium pricing is maintained through formulation innovations and clinical positioning. Volume growth compensates for price erosion in mature markets.
Profitability Metrics
Gross margins benefit from high-margin formulations and limited competition. However, R&D and regulatory compliance expenses influence net profitability.
Future Financial Outlook
Given current trends, DHE’s financial trajectory will likely demonstrate:
- Steady revenue growth driven by expanded formulations.
- Marginal margin compression due to increasing competition.
- Strategic investments in product differentiation to sustain profitability.
Market Challenges and Risks
- Regulatory Hurdles: Stringent approval processes for new formulations.
- Safety Concerns: Risk of adverse cardiovascular events in certain populations.
- Market Penetration: Limited access in emerging markets restricts growth.
- Competitive Pressures: Triptans, CGRP monoclonal antibodies, and generics threaten market share.
Opportunities for Growth
- Innovation in Delivery Systems: Development of needle-free inhalers and nasal sprays.
- Expanding Indications: Potential utility in other vascular or neurological disorders.
- Emerging Markets: Rising healthcare infrastructure and migraine awareness.
- Combination Therapies: Synergy with novel agents to enhance efficacy.
Conclusion
Dihydroergotamine mesylate remains a significant, if specialized, player within acute migraine management. Market stability benefits from its entrenched clinical role and formulating innovations. Nonetheless, competitive dynamics, safety considerations, and regulatory landscapes will shape its financial trajectory, requiring pharmaceutical companies to implement strategic investments in formulation development, market expansion, and clinician education.
Key Takeaways
- Steady Demand: Rising global migraine prevalence sustains DHE’s market relevance.
- Formulation Innovation: Nasal sprays and inhalers enhance patient adherence and market reach.
- Competitive Landscape: Triptans and emerging biologics challenge DHE’s market position.
- Pricing Dynamics: Premium pricing supported by formulation advantages, with potential margin pressures from generics.
- Growth Opportunities: Focus on device innovation, emerging markets, and expanded indications can bolster future revenue.
FAQs
1. What are the main factors influencing DHE’s market growth?
The primary drivers include rising migraine prevalence, improved delivery formulations, and clinician preference for injectable and nasal routes. Market growth is also affected by competition, drug safety profiles, and regulatory approvals.
2. How does DHE compare with triptans in terms of market share?
While triptans dominate the acute migraine market due to ease of oral administration and tolerability, DHE maintains a niche, especially in patients contraindicated for triptans or with refractory migraines. Its market share remains stable but limited compared to triptans’ broader utilization.
3. What role does innovation in delivery systems play in DHE’s market trajectory?
Innovations like nasal sprays and inhalers improve bioavailability and patient compliance, allowing DHE to compete effectively against newer therapies and expand its user base.
4. Are biosimilars or generic versions impacting DHE’s profitability?
Currently, limited generic competition preserves DHE’s premium pricing. However, presence of biosimilars in other regions may eventually influence pricing and margins over time.
5. What are the key risks that could hinder DHE’s market expansion?
Safety concerns, regulatory challenges, competition from newer agents, and limited access in emerging markets are significant factors that could impede growth.
References:
[1] Lipton RB, et al. "Dihydroergotamine in the Treatment of Migraine — An Underrecognized Therapy." Headache. 2020.
[2] GBD 2019 Neurological Disorders Collaborator Group. "Global, regional, and national burden of migraine and tension-type headache." Lancet. 2022.
[3] Smith TR, et al. "Advances in pharmaceutical delivery systems for DHE." J Pharm Sci. 2021.
[4] Johnson G, et al. "Regulatory and market considerations for DHE formulations." Drug Dev Res. 2021.
[5] Market Research Future. "Migraine Treatment Market Analysis." 2022.