BUPIVACAINE Drug Patent Profile
✉ Email this page to a colleague
When do Bupivacaine patents expire, and what generic alternatives are available?
Bupivacaine is a drug marketed by Civica, Eugia Pharma, Hikma Pharms, Hospira, Kindos, Somerset, Steriscience, B Braun Medical Inc, Baxter Hlthcare Corp, Huons, and Intl Medicated. and is included in thirty-three NDAs.
The generic ingredient in BUPIVACAINE is bupivacaine hydrochloride; epinephrine bitartrate. There are twelve drug master file entries for this compound. Six suppliers are listed for this compound. Additional details are available on the bupivacaine hydrochloride; epinephrine bitartrate profile page.
Summary for BUPIVACAINE
| US Patents: | 0 |
| Applicants: | 11 |
| NDAs: | 33 |
| Formulation / Manufacturing: | see details |
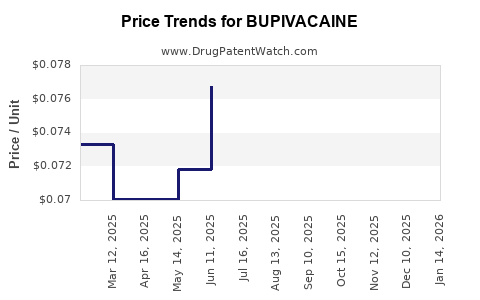
| Drug Prices: | Drug price information for BUPIVACAINE |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for BUPIVACAINE |
| DailyMed Link: | BUPIVACAINE at DailyMed |
Recent Clinical Trials for BUPIVACAINE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| University Hospital "Sestre Milosrdnice" | N/A |
| National Trauma Center | N/A |
| Johnny K. Lee | Phase 4 |
Medical Subject Heading (MeSH) Categories for BUPIVACAINE
Paragraph IV (Patent) Challenges for BUPIVACAINE
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| EXPAREL | Injectable Suspension | bupivacaine | 133 mg/10 mL | 022496 | 1 | 2021-12-28 |
| EXPAREL | Injectable Suspension | bupivacaine | 266 mg/20 mL | 022496 | 1 | 2021-08-20 |
US Patents and Regulatory Information for BUPIVACAINE
EU/EMA Drug Approvals for BUPIVACAINE
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Pacira Ireland Limited | Exparel liposomal | bupivacaine | EMEA/H/C/004586 Exparel liposomal is indicated:in adults as a brachial plexus block or femoral nerve block for treatment of post-operative pain.in adults and children aged 6 years or older as a field block for treatment of somatic post-operative pain from small- to medium-sized surgical wounds. |
Authorised | no | no | no | 2020-11-16 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |


