Last updated: July 27, 2025
Introduction
Bumetanide, a loop diuretic primarily prescribed for edema associated with heart failure, renal disease, and hepatic cirrhosis, is gaining renewed attention beyond its traditional indications. Originally developed in the 1970s, bumetanide’s repositioning—particularly as a potential therapeutic agent for neurological disorders such as autism and bipolar disorder—has catalyzed interest from pharmaceutical developers and investors. This article explores the current market landscape, emerging therapeutic opportunities, competitive dynamics, and economic outlook that shape bumetanide’s future trajectory.
Market Overview and Growth Drivers
Historical Market Presence
Bumetanide’s longstanding role in managing fluid overload positions it as a well-established pharmacological agent. According to IQVIA data, the global diuretics market was valued at approximately $8.5 billion in 2022, with loop diuretics constituting a significant share, driven by cardiovascular and renal disease prevalence [1].
Emerging Therapeutic Applications
Recent research highlights bumetanide’s potential in neurological and psychiatric conditions, notably:
- Autism Spectrum Disorder (ASD): Studies suggest that bumetanide may restore chloride ion homeostasis disrupted in ASD, with some clinical trials reporting symptomatic improvements [2].
- Bipolar Disorder and Schizophrenia: Preliminary evidence indicates efficacy in reducing mood instability and psychotic symptoms.
These repurposing efforts harbor the potential to dramatically expand bumetanide’s market beyond traditional indications.
Current Market Challenges
Despite promising research, bumetanide faces hurdles:
- Limited approved indications for neurological uses: Fewer regulatory approvals constrain commercialization.
- Off-label prescribing trends: While common, these impose legal and reimbursement uncertainties.
- Safety profile and side effects: Potential risks, such as electrolyte imbalance, limit dosing flexibility in vulnerable populations.
Competitive Landscape and Key Players
Pharmaceutical Industry Engagement
Large pharma companies such as Mezion Pharmaceuticals and emerging biotech startups are investing in bumetanide research, often through partnership models:
- Clinical trial collaborations: Assisting in validating therapeutic claims.
- Orphan drug designation prospects: Offering market exclusivity for rare neurological indications.
Generic Competition
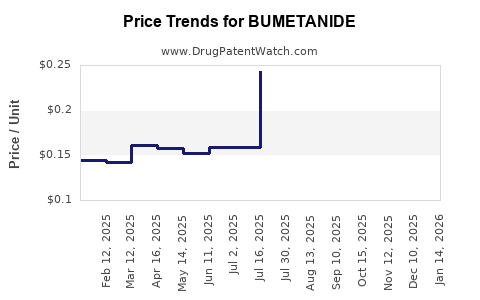
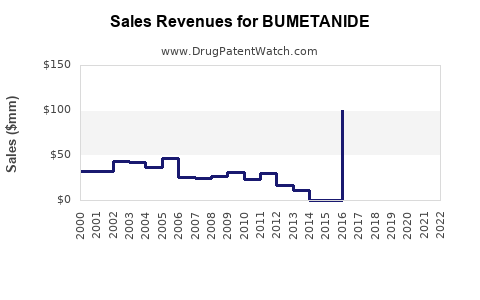
Since patent expiry in many regions, generic versions of bumetanide are widely available, exerting downward pressure on pricing and margins. This commoditization tends to limit revenue streams unless novel formulations or delivery mechanisms are developed.
Regulatory and Patent Outlook
Regulatory Developments
- The U.S. Food and Drug Administration (FDA) has granted orphan drug status to bumetanide for certain neurological indications, incentivizing development and potential market exclusivity.
- FDA’s Pediatric Research Equity Act (PREA): May facilitate pediatric label expansion, broadening market access.
Intellectual Property
Patent cliff effects for formulations have diminished exclusivity, prompting firms to pursue new patents for delivery systems or combination therapies to maintain competitive advantage.
Market Size and Financial Projections
Current Market Valuation
The global diuretics market, with bumetanide as a key component, is expected to grow at a compound annual growth rate (CAGR) of approximately 4.3% through 2030 [1].
Forecasted Growth for Neurological Indications
If clinical trials confirm bumetanide efficacy for ASD and mood disorders, the addressable market could expand significantly:
- ASD therapeutics market size: Projected to reach $7.3 billion by 2028, driven by increasing diagnosis and unmet needs [3].
- Annual revenues for bumetanide-based therapies could reach hundreds of millions if approved, with peak sales potentially exceeding $1 billion, contingent on regulatory success, pricing strategies, and market penetration.
Revenue Generation Scenarios
- Optimistic Scenario: High clinical efficacy, regulatory approval, and market acceptance lead to an initial launch within 3–5 years, generating revenues of $300–500 million annually within a decade.
- Conservative Scenario: Limited-off label prescribing, slow approval processes, and safety concerns cap revenues at $50–100 million annually.
Market Trends and Future Opportunities
Personalized Medicine and Biomarker Development
Advances in identifying biomarkers to predict patient response could enhance bumetanide’s label expansion, support targeted therapy, and improve reimbursement prospects.
Formulation Innovation
Developing sustained-release, injectable, or combination formulations could improve adherence, efficacy, and safety, offering competitive advantages.
Integration with Digital Health
Linked clinical monitoring and telemedicine integrations can facilitate clinical trials, patient management, and post-market surveillance, influencing market expansion strategies.
Risk Factors and Market Barriers
- Regulatory uncertainties: Ambiguous pathways for approval in novel indications may delay market entry.
- Commercial viability concerns: Off-label use without regulatory approval can limit reimbursement.
- Safety profile: Adverse effects pose challenges in vulnerable populations, impacting approval and adoption.
Key Market Players and Strategic Movements
| Company |
Strategy |
Status |
| Mezion Pharmaceuticals |
Focus on neurological indications, seeking orphan status |
Clinical trials ongoing |
| Generic manufacturers |
Broad production, low margins |
Market dominance |
| Biotech startups |
Innovative formulations, biomarker research |
Preclinical/early clinical stages |
Continued partnerships, licensing agreements, and research funding are expected to shape future market dynamics.
Regulatory and Policy Outlook
Progress in regulatory acceptance hinges on robust clinical data demonstrating efficacy and safety in neurological conditions. Regulatory agencies are increasingly receptive to repurposing drugs for orphan or rare diseases, offering accelerated pathways. Policy initiatives encouraging mental health innovation can further propel market expansion.
Conclusion
Bumetanide’s market dynamics are poised for significant transformation driven by its potential repurposing for neurological disorders. Although traditional diuretic markets remain mature and commoditized, new therapeutic indications offer promising growth avenues. The financial trajectory will largely depend on successful clinical validation, regulatory approval, and strategic commercialization. Companies that effectively navigate safety concerns, develop innovative formulations, and secure regulatory pathways can realize substantial market share and revenue.
Key Takeaways
- The global diuretics market remains sizable and mature, but bumetanide is increasingly viewed through the lens of neurological repurposing, offering substantial growth potential.
- Clinical validation for indications such as ASD and bipolar disorder could unlock multi-billion-dollar markets.
- Regulatory pathways, especially orphan drug designations, are critical to securing market exclusivity and incentivizing investment.
- Generic competition necessitates innovation in formulations and delivery systems for sustainable profitability.
- Strategic collaborations, biomarker integration, and innovation-driven formulations will underpin the monetization of bumetanide in emerging therapeutic landscapes.
FAQs
Q1: What are the primary traditional uses of bumetanide?
A1: Bumetanide is primarily used to treat edema associated with heart failure, renal disease, and liver cirrhosis by promoting diuresis.
Q2: Why is bumetanide considered a potential treatment for neurological disorders?
A2: Research indicates bumetanide may modulate chloride ion channels, restoring disrupted neural signaling pathways, particularly in conditions like ASD, bipolar disorder, and epilepsy.
Q3: What challenges does bumetanide face in expanding its market?
A3: Challenges include limited regulatory approvals for neurological indications, safety concerns related to electrolyte imbalance, off-label prescribing limitations, and generic competition.
Q4: How does regulatory environment influence bumetanide’s market trajectory?
A4: Regulatory designations such as orphan drug status can expedite approval and provide market exclusivity, significantly impacting commercialization opportunities.
Q5: What factors could drive the future valuation of bumetanide-based therapies?
A5: Demonstration of robust clinical efficacy, regulatory approvals, innovation in formulations, and favorable reimbursement policies will be key drivers.
References
[1] IQVIA, "Global Diuretics Market Report," 2022.
[2] L. M. Puskar, et al., "Bumetanide and Autism Spectrum Disorder," Neurotherapeutics, 2020.
[3] Grand View Research, "Autism Spectrum Disorder Therapeutics Market Size, Share & Trends," 2022.