BRAFTOVI Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Braftovi, and what generic alternatives are available?
Braftovi is a drug marketed by Array Biopharma Inc and is included in one NDA. There are thirteen patents protecting this drug and one Paragraph IV challenge.
This drug has one hundred and ninety-eight patent family members in fifty-three countries.
The generic ingredient in BRAFTOVI is encorafenib. One supplier is listed for this compound. Additional details are available on the encorafenib profile page.
DrugPatentWatch® Generic Entry Outlook for Braftovi
Braftovi was eligible for patent challenges on June 27, 2022.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be August 5, 2033. This may change due to patent challenges or generic licensing.
There have been ten patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for BRAFTOVI?
- What are the global sales for BRAFTOVI?
- What is Average Wholesale Price for BRAFTOVI?
Summary for BRAFTOVI
| International Patents: | 198 |
| US Patents: | 13 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 59 |
| Clinical Trials: | 20 |
| Patent Applications: | 2,502 |
| Drug Prices: | Drug price information for BRAFTOVI |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for BRAFTOVI |
| What excipients (inactive ingredients) are in BRAFTOVI? | BRAFTOVI excipients list |
| DailyMed Link: | BRAFTOVI at DailyMed |
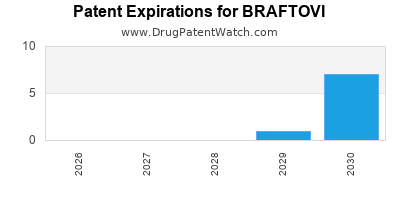

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for BRAFTOVI
Generic Entry Date for BRAFTOVI*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
CAPSULE;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for BRAFTOVI
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| National Cancer Institute (NCI) | Phase 1 |
| Deciphera Pharmaceuticals LLC | Phase 1/Phase 2 |
| Pfizer | Phase 2 |
Paragraph IV (Patent) Challenges for BRAFTOVI
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| BRAFTOVI | Capsules | encorafenib | 75 mg | 210496 | 3 | 2022-06-27 |
US Patents and Regulatory Information for BRAFTOVI
BRAFTOVI is protected by thirty-one US patents and four FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of BRAFTOVI is ⤷ Get Started Free.
This potential generic entry date is based on patent 9,474,754.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Array Biopharma Inc | BRAFTOVI | encorafenib | CAPSULE;ORAL | 210496-002 | Jun 27, 2018 | RX | Yes | Yes | 8,946,250 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Array Biopharma Inc | BRAFTOVI | encorafenib | CAPSULE;ORAL | 210496-002 | Jun 27, 2018 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Array Biopharma Inc | BRAFTOVI | encorafenib | CAPSULE;ORAL | 210496-002 | Jun 27, 2018 | RX | Yes | Yes | 9,593,100 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Array Biopharma Inc | BRAFTOVI | encorafenib | CAPSULE;ORAL | 210496-002 | Jun 27, 2018 | RX | Yes | Yes | 10,258,622 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Array Biopharma Inc | BRAFTOVI | encorafenib | CAPSULE;ORAL | 210496-001 | Jun 27, 2018 | DISCN | Yes | No | 9,314,464 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Array Biopharma Inc | BRAFTOVI | encorafenib | CAPSULE;ORAL | 210496-002 | Jun 27, 2018 | RX | Yes | Yes | 9,850,230 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for BRAFTOVI
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Pierre Fabre Medicament | Braftovi | encorafenib | EMEA/H/C/004580Encorafenib is indicated:in combination with binimetinib is indicated for the treatment of adult patients with unresectable or metastatic melanoma with a BRAF V600 mutationin combination with cetuximab, for the treatment of adult patients with metastatic colorectal cancer (CRC) with a BRAF V600E mutation, who have received prior systemic therapy | Authorised | no | no | no | 2018-09-19 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for BRAFTOVI
When does loss-of-exclusivity occur for BRAFTOVI?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 2045
Patent: COMBINACIONES FARMACEUTICAS
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 13299841
Patent: Pharmaceutical combinations comprising a B-Raf inhibitor, an EGFR inhibitor and optionally a PI3K-alpha inhibitor
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2015002384
Patent: combinações farmacêuticas compreendendo um inibidor de b-raf, um inibidor de egfr e opcionalmente um inibidor de pi3k-alfa
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 79548
Patent: COMBINAISONS PHARMACEUTIQUES COMPRENANT UN INHIBITEUR DE B-RAF, UN INHIBITEUR D'EGFR ET FACULTATIVEMENT UN INHIBITEUR DE PI3K ALPHA (PHARMACEUTICAL COMBINATIONS COMPRISING A B-RAF INHIBITOR, AN EGFR INHIBITOR AND OPTIONALLY A PI3K-ALPHA INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 15000294
Patent: Combinaciones farmaceuticas que comprenden un inhibidor de b-raf, un inhibidor del receptor del factor de crecimiento epidermico y opcionalmente un inhibidor pi3halpha y uso en el tratamiento de una enfermedad proliferativa.
Estimated Expiration: ⤷ Get Started Free
China
Patent: 4519887
Patent: Pharmaceutical combinations comprising a b-raf inhibitor, an egfr inhibitor and optionally a pi3k-alpha inhibitor
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 00273
Patent: Combinaciones farmaceúticas que comprenden un inhibidor de b-raf, un inhibidor de egfr y opcionalmente un inhibidor de pi3k-alfa
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0190537
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 22143
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 82440
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 15008695
Patent: COMBINACIONES FARMACÉUTICAS QUE COMPRENDEN UN INHIBIDOR DE B-RAF, UN INHIBIDOR DE EGFR Y OPCIONALMENTE UN INHIBIDOR DE PI3K-ALFA
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 8420
Patent: ФАРМАЦЕВТИЧЕСКИЕ КОМБИНАЦИИ, СОДЕРЖАЩИЕ ИНГИБИТОР B-RAF, ИНГИБИТОР EGFR И, НЕОБЯЗАТЕЛЬНО, ИНГИБИТОР PI3K-АЛЬФА (PHARMACEUTICAL COMBINATIONS COMPRISING A B-Raf INHIBITOR, AN EGFR INHIBITOR AND OPTIONALLY A PI3K-ALPHA INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Patent: 1590332
Patent: ФАРМАЦЕВТИЧЕСКИЕ КОМБИНАЦИИ, СОДЕРЖАЩИЕ ИНГИБИТОР B-RAF, ИНГИБИТОР EGFR И, НЕОБЯЗАТЕЛЬНО, ИНГИБИТОР PI3K-АЛЬФА
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 82440
Patent: COMBINAISONS DE MEDICAMENTS CONTENANT UN INHIBITEUR DE B-RAF, UN INHIBITEUR DE L'EGFR, ET OPTIONELLEMENT UN INHIBITEUR PI3K-ALPHA (PHARMACEUTICAL COMBINATIONS COMPRISING A B-RAF INHIBITOR, AN EGFR INHIBITOR AND OPTIONALLY A PI3K-ALPHA INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Patent: 74904
Patent: COMBINAISONS PHARMACEUTIQUES COMPRENANT UN INHIBITEUR B-RAF, UN INHIBITEUR D'EGFR ET ÉVENTUELLEMENT UN INHIBITEUR PI3K-ALPHA (PHARMACEUTICAL COMBINATIONS COMPRISING A B-RAF INHIBITOR, AN EGFR INHIBITOR AND OPTIONALLY A PI3K-ALPHA INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Guatemala
Patent: 1500025
Patent: COMBINACIONES FARMACÉUTICAS QUE COMPRENDEN UN INHIBIDOR DE B-RAF, UN INHIBIDOR DE EGFR Y OPCIONALMENTE UN INHIBIDOR DE PI3K-ALFA
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 04976
Patent: 種包含 抑制劑, 抑制劑,及視需要 抑制劑的醫藥組合 (PHARMACEUTICAL COMBINATIONS COMPRISING A B-RAF INHIBITOR, AN EGFR INHIBITOR AND OPTIONALLY A PI3K-ALPHA INHIBITOR B-RAF EGFR PI3K-ALPHA)
Estimated Expiration: ⤷ Get Started Free
Patent: 11831
Patent: 包含 抑制劑、 抑制劑和任選 α抑制劑的藥物組合 (PHARMACEUTICAL COMBINATIONS COMPRISING A B-RAF INHIBITOR, AN EGFR INHIBITOR AND OPTIONALLY A PI3K-ALPHA INHIBITOR B-RAF EGFR PI3K)
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 42877
Estimated Expiration: ⤷ Get Started Free
India
Patent: 0DEN2015
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 6934
Patent: תכשיר רוקחי המכיל מעכב b-raf, מעכב egfr ומעכב pi3k-alpha אופציונאלי (Pharmaceutical combinations comprising a b-raf inhibitor, an egfr inhibitor and optionally a pi3k-alpha inhibitor)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 42396
Estimated Expiration: ⤷ Get Started Free
Patent: 95024
Estimated Expiration: ⤷ Get Started Free
Patent: 74669
Estimated Expiration: ⤷ Get Started Free
Patent: 15524472
Patent: B−Raf阻害薬、EGFR阻害薬及び場合によってはPI3K−α阻害薬を含む組合せ医薬
Estimated Expiration: ⤷ Get Started Free
Patent: 18109022
Patent: B−Raf阻害薬、EGFR阻害薬及び場合によってはPI3K−α阻害薬を含む組合せ医薬 (PHARMACEUTICAL COMBINATIONS COMPRISING B-Raf INHIBITOR, AND EGFR INHIBITOR AND OPTIONALLY PI3K-α INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Patent: 20019780
Patent: B−Raf阻害薬、EGFR阻害薬及び場合によってはPI3K−α阻害薬を含む組合せ医薬 (PHARMACEUTICAL COMBINATIONS COMPRISING B-RAF INHIBITOR, AND EGFR INHIBITOR AND OPTIONALLY PI3K-α INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Jordan
Patent: 0130236
Patent: تركيبات صيدلانية تشتمل على مثبط B-RAF ومثبط EGFR ومثبط اختياري لــPI3K ALPHA (PHARMACEUTICAL COMBINATIONS COMPRISING A B-RAF INHIBITOR, AN EGFR INHIBITOR AND OPTIONALLY A PI3K-ALPHA INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 82440
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 6031
Patent: PHARMACEUTICAL COMBINATIONS COMPRISING A B-RAF INHIBITOR, AN EGFR INHIBITOR AND OPTIONALLY A PI3K-ALPHA INHIBITOR
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 9403
Patent: COMBINACIONES FARMACÉUTICAS QUE COMPRENDEN UN INHIBIDOR DE B-RAF, UN INHIBIDOR DE EGFR, Y OPCIONALMENTE UN INHIBIDOR DE PI3K-ALFA. (PHARMACEUTICAL COMBINATIONS COMPRISING A B-RAF INHIBITOR, AN EGFR INHIBITOR AND OPTIONALLY A PI3K-ALPHA INHIBITOR.)
Estimated Expiration: ⤷ Get Started Free
Patent: 15001732
Patent: COMBINACIONES FARMACEUTICAS QUE COMPRENDEN UN INHIBIDOR DE B-RAF, UN INHIBIDOR DE EGFR, Y OPCIONALMENTE UN INHIBIDOR DE P13K-ALFA. (PHARMACEUTICAL COMBINATIONS COMPRISING A B-RAF INHIBITOR, AN EGFR INHIBITOR AND OPTIONALLY A PI3K-ALPHA INHIBITOR.)
Estimated Expiration: ⤷ Get Started Free
Morocco
Patent: 829
Patent: Combinaisons pharmaceutiques comprenant un inhibiteur de b-raf, un inhibiteur d'egfr et facultativement un inhibiteur de pi3k alpha
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 3940
Patent: Pharmaceutical combinations comprising a b-raf inhibitor, an egfr inhibitor and optionally a pi3k-alpha inhibitor
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 150673
Patent: COMBINACIONES FARMACEUTICAS
Estimated Expiration: ⤷ Get Started Free
Patent: 191655
Patent: COMBINACIONES FARMACEUTICAS
Estimated Expiration: ⤷ Get Started Free
Philippines
Patent: 015500246
Patent: PHARMACEUTICAL COMBINATIONS COMPRISING A B-RAF INHIBITOR, AN EGFR INHIBITOR AND OPTIONALLY A PI3K-ALPHA INHIBITOR
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 82440
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 82440
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 734
Patent: FARMACEUTSKE KOMBINACIJE KOJE OBUHVATAJU B-RAF INHIBITOR, EGFR INHIBITOR I OPCIONO PI3K-ALFA INHIBITOR (PHARMACEUTICAL COMBINATIONS COMPRISING A B-RAF INHIBITOR, AN EGFR INHIBITOR AND OPTIONALLY A PI3K-ALPHA INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 201500321Y
Patent: PHARMACEUTICAL COMBINATIONS COMPRISING A B-RAF INHIBITOR, AN EGFR INHIBITOR AND OPTIONALLY A PI3K-ALPHA INHIBITOR
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 82440
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 2112885
Estimated Expiration: ⤷ Get Started Free
Patent: 150040905
Patent: B-RAF 억제제, EGFR 억제제 및 임의로 PI3K-알파 억제제를 포함하는 제약 조합물 (PHARMACEUTICAL COMBINATIONS COMPRISING A B-RAF INHIBITOR, AN EGFR INHIBITOR AND OPTIONALLY A PI3K-ALPHA INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 17911
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 07754
Estimated Expiration: ⤷ Get Started Free
Patent: 1410247
Patent: Pharmaceutical combinations
Estimated Expiration: ⤷ Get Started Free
Tunisia
Patent: 15000027
Patent: PHARMACEUTICAL COMBINATIONS COMPRISING A B-RAF INHIBITOR, AN EGFR INHIBITOR AND OPTIONALLY A PI3K-ALPHA INHIBITOR
Estimated Expiration: ⤷ Get Started Free
Turkey
Patent: 1904980
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 5786
Patent: ФАРМАЦЕВТИЧНА КОМБІНАЦІЯ, ЩО МІСТИТЬ ІНГІБІТОР B-Raf, ІНГІБІТОР EGFR ТА, НЕОБОВ'ЯЗКОВО, ІНГІБІТОР РІ3K-? (PHARMACEUTICAL COMBINATIONS COMPRISING A B-RAF INHIBITOR, AN EGFR INHIBITOR AND OPTIONALLY A PI3K-ALPHA INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering BRAFTOVI around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Hong Kong | 1197024 | 醫藥製劑 (PHARMACEUTICAL FORMULATIONS) | ⤷ Get Started Free |
| Taiwan | I649098 | ⤷ Get Started Free | |
| Slovenia | 2882440 | ⤷ Get Started Free | |
| Hungary | E032847 | ⤷ Get Started Free | |
| Brazil | 112014011981 | formulações farmacêuticas | ⤷ Get Started Free |
| Ecuador | SP23034537 | FORMULACIONES FARMACEUTICAS | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for BRAFTOVI
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2470526 | 122019000021 | Germany | ⤷ Get Started Free | PRODUCT NAME: ENCORAFENIB EINSCHLIESSLICH ENCORAFENIB IN FORM EINES PHARMAZEUTISCH VERTRAEGLICHEN SALZES ODER SOLVATS; REGISTRATION NO/DATE: EU/1/18/1314 20180920 |
| 2470526 | SPC/GB19/009 | United Kingdom | ⤷ Get Started Free | PRODUCT NAME: ENCORAFENIB OR PHARMACEUTICALLY ACCEPTABLE SALTS OR SOLVATES THEREOF; REGISTERED: UK EU/1/18/1314/001-002 20180924; UK PLGB 00603/0240-0001 20180924; UK PLGB 00603/0241-0001 20180924 |
| 2727918 | PA2019006 | Lithuania | ⤷ Get Started Free | PRODUCT NAME: BINIMETINIBO IR ENKORAFENIBO, KIEKVIENO BET KURIOMIS FORMOMIS, KURIOMS TAIKOMA PAGRINDINIO PATENTO APSAUGA, DERINYS; REGISTRATION NO/DATE: EU/1/18/1314, EU/1/18/1835 20190920 |
| 2727918 | LUC00102 | Luxembourg | ⤷ Get Started Free | PRODUCT NAME: COMBINAISON DE BINIMETINIB ET D'ENCORAFENIB, CHACUN SOUS TOUTES SES FORMES TELLES QUE PROTEGEES PAR LE BREVET DE BASE; AUTHORISATION NUMBER AND DATE: EU/1/18/1314 20180924 |
| 2727918 | C20190009 | Finland | ⤷ Get Started Free | TZ1Y, PARTY DATA CHANGE RELATED TO A GRANTED SPC |
| 2470526 | 300973 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: ENCORAFENIB; REGISTRATION NO/DATE: EU/1/18/1314 20180924 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for BRAFTOVI (Encorafenib)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
