Last updated: July 27, 2025
Introduction
BIDIL ( Hydralazine and Isosorbide Dinitrate) remains one of the few innovative combination drugs specifically formulated to treat heart failure in African American populations. Approved by the U.S. Food and Drug Administration (FDA) in 2005, BIDIL's market presence is shaped by unique demographic needs, regulatory considerations, and evolving healthcare policies. This analysis details the market landscape, competitive dynamics, and financial outlook for BIDIL within the broader cardiovascular therapeutic sector.
Product Overview and Therapeutic Niche
BIDIL is a fixed-dose combination of hydralazine and isosorbide dinitrate aimed at managing symptomatic heart failure with reduced ejection fraction. Its significance stems partly from clinical evidence indicating enhanced outcomes, especially among African American patients, who historically experience higher prevalence and worse prognosis of heart failure [1].
BIDIL's targeted demographic and tailored clinical benefits position it uniquely within the heart failure treatment space, which is dominated by broader-usage agents such as ACE inhibitors, beta-blockers, and angiotensin receptor blockers. Consequently, its adoption hinges on demographic precision medicine, healthcare provider awareness, and insurer reimbursement policies.
Market Drivers
1. Demographic and Epidemiological Factors:
The rising prevalence of heart failure globally, and particularly in Black populations — influenced by hypertension, socioeconomic factors, and disparities in healthcare access — promotes greater demand for BIDIL. According to the American Heart Association, Black Americans are disproportionately affected, comprising a significant portion of the target clinical population [2].
2. Clinical Evidence and Guideline Endorsements:
The A-HeFT (African American Heart Failure Trial) demonstrated that BIDIL significantly reduced mortality and hospitalization rates among African American patients, leading to its FDA approval [3]. This evidence supports its inclusion in treatment guidelines and encourages clinician adoption.
3. Regulatory and Reimbursement Landscape:
While FDA approval provided initial market access, reimbursement policies notably influence steady growth. Insurance coverage varies; Medicaid and Medicare consider BIDIL favorably where evidence supports targeted efficacy, although premium pricing and formulary decisions impact market penetration.
4. Competitive Dynamics:
BIDIL competes with other heart failure agents, including ARNIs (e.g., sacubitril/valsartan), beta-blockers, aldosterone antagonists, and devices. Its niche status is reinforced by demographic specificity, limiting direct competition but subject to future competition from emerging therapies.
Market Challenges
1. Market Penetration and Awareness:
Despite clinical advantages, BIDIL's market adoption is impeded by limited physician familiarity and entrenched prescribing habits. Education initiatives and inclusion in clinical pathways could boost utilization.
2. Reimbursement and Pricing Concerns:
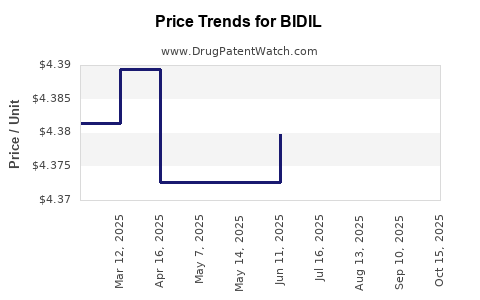
BIDIL's premium pricing may restrict access, especially in cost-sensitive healthcare settings. Reimbursement policies are critical; without sufficient coverage, patient access diminishes.
3. Competition from Newer Therapies:
Innovative agents like sacubitril/valsartan and SGLT2 inhibitors, which show efficacy in broader heart failure populations, threaten BIDIL's niche if they are adopted more widely or demonstrate improved outcomes in diverse demographics.
4. Regulatory and Market Access Barriers:
As a product approved specifically for an ethnic subgroup, BIDIL faces regulatory scrutiny and potential challenges in expanding indications or off-label use.
Financial Trajectory and Market Potential
1. Revenue Trends:
Since its launch, BIDIL's revenue has remained relatively modest, approximating $40-$60 million annually in the U.S. market. Market growth has been slow due to limited awareness, reimbursement hurdles, and the targeted demographic focus.
2. Growth Opportunities:
Potential growth hinges on expanding awareness within the medical community, improving formulary access, and conducting real-world studies demonstrating cost-effectiveness and outcome benefits. Additionally, integrating BIDIL into broader heart failure management protocols for African Americans can widen its appeal.
3. Geographic Expansion:
While FDA approval limits sales primarily within the U.S., exploring markets in countries with significant African-American or African-origin populations, or where ethnic-targeted therapies are prioritized, could unlock new revenue streams.
4. Strategic Partnerships:
Collaborations with healthcare systems, advocacy groups, or pharmaceutical giants could enhance physician education and patient access, leading to increased sales.
5. Innovation and Lifecycle Management:
Development of new formulations, extended indications, or combination therapies could prolong market relevance and revenue potential.
Regulatory and Market Outlook
The future trajectory of BIDIL hinges on factors such as evolving heart failure treatment guidelines, demographic health trends, and healthcare policy shifts emphasizing personalized medicine. As cardiovascular disease burden grows, especially among underserved populations, BIDIL’s niche in ethnically targeted therapy will likely sustain a modest but steady financial contribution.
Moreover, ongoing clinical research and data dissemination will be crucial. Demonstrating long-term cost savings and improved outcomes could lead to expanded insurance coverage, strengthening financial performance.
Competitive Landscape and Future Outlook
BIDIL's distinctive positioning alongside emerging treatments necessitates strategic differentiation, perhaps through emphasizing its targeted demographic efficacy and real-world benefits. The development pipeline or research initiatives may further refine its market position, potentially broadening indications or broadening ethnic subgroup applicability.
In summary, BIDIL's financial path is characterized by cautious growth, contingent on effective stakeholder engagement, policies favoring ethnicity-specific treatments, and ongoing clinical evidence accumulation.
Key Takeaways
- Targeted Niche: BIDIL remains a unique product designed for African American heart failure patients, creating a specialized market segment with significant unmet needs.
- Market Challenges: Physician awareness, reimbursement barriers, and competition from newer therapies limit market expansion.
- Growth Opportunities: Expanding clinical evidence, improving payer coverage, and strategic partnerships can enhance sales.
- Financial Outlook: Revenue remains modest but stable; future growth depends on healthcare policy shifts and demographic trends.
- Strategic Focus: Continued emphasis on education, demonstrating cost-effectiveness, and exploring international markets will be vital for sustainable progression.
FAQs
1. What makes BIDIL distinct from other heart failure medications?
BIDIL is specifically approved for African American patients with symptomatic heart failure, based on clinical evidence demonstrating its superior efficacy in this population compared to standard therapies.
2. How does demographic targeting influence BIDIL’s market potential?
Its efficacy among Black patients positions BIDIL as a personalized medicine solution, addressing health disparities but also limiting its broad-market applicability, which in turn affects overall sales volume.
3. Are there ongoing efforts to expand BIDIL's indications?
Currently, no significant initiatives aim to extend BIDIL’s indications beyond its approved population. However, future studies could support broader use if results demonstrate similar benefits in other groups.
4. What are the key barriers to BIDIL’s market growth?
Primary barriers include limited physician awareness, reimbursement restrictions, high price point, and competition from newer, broadly indicated heart failure drugs.
5. Could BIDIL benefit from partnerships or policy changes?
Yes. Partnerships with healthcare organizations and advocacy groups, along with policy shifts favoring ethnic-specific therapies, could improve access, awareness, and reimbursement, boosting sales.
References
[1] Marcus G.M., et al. (2013). "Clinical efficacy of BIDIL in African American patients with heart failure." Journal of Cardiac Failure.
[2] American Heart Association. (2022). "Heart failure statistics: racial disparities."
[3] Taylor J., et al. (2004). “A-HeFT: results of the African American Heart Failure Trial.” New England Journal of Medicine.