Last updated: December 31, 2025
Executive Summary
Benztropine mesylate, a cholinergic antagonist primarily used to treat Parkinson's disease and extrapyramidal symptoms, has maintained a stable niche within neurological therapeutics. While its market size remains relatively modest compared to blockbuster drugs, recent trends such as increased neurological disease prevalence and regulatory shifts are shaping its market dynamics. This article explores the current market status, growth drivers, challenges, and future outlook for benztropine mesylate, supported by quantitative data, key stakeholders, and policy considerations.
What Is Benztropine Mesylate?
| Attribute |
Details |
| Chemical Name |
1-phenyl-3-(tropan-3-yl)propan-1-one mesylate |
| Mechanism of Action |
Centrally acting anticholinergic; reduces tremors and rigidity by balancing dopamine and acetylcholine in the brain |
| Approved Uses |
Parkinson's disease, drug-induced extrapyramidal symptoms |
| Formulations |
Injectable, oral tablets |
Market Overview
Global Market Size and Revenue
| Indicator |
Data (USD Millions) |
Year |
Source |
| Global Pharmaceutical Market (Neurology Segment) |
75,000 |
2022 |
IQVIA[1] |
| Benztropine Market Share |
150 |
2022 |
Estimated |
| Estimated Market Value (2023) |
165 |
Projected |
With a CAGR of 4% |
Market Share Context
- Market Penetration: Benztropine accounts for approximately 0.2% of the global neurology therapeutic market.
- Dominant Competitors: Other antiparkinsonian agents like levodopa, dopamine agonists, and COMT inhibitors dominate the market, limiting benztropine's growth.
Regional Distribution
| Region |
Market Share |
Key Drivers |
Challenges |
| North America |
45% |
Established healthcare infrastructure, high PD prevalence |
Patent expirations, regulatory constraints |
| Europe |
30% |
Aging population, healthcare policies |
Pricing pressures |
| Asia-Pacific |
20% |
Increasing neurodegeneration cases, emerging markets |
Limited access, regulatory variance |
Market Dynamics
Growth Drivers
| Driver |
Impact |
Evidence/Statistics |
| Rising Neurodegenerative Disease Burden |
Drives demand for symptomatic treatments including benztropine |
WHO projects a 40% rise in Parkinson's prevalence by 2040[2] |
| Regulatory Approvals & Off-label Use |
Expansion in off-label indications and formulations |
Nascent research into pediatric extrapyramidal symptoms |
| Pricing & Reimbursement Policies |
Favorability in select markets increases accessibility |
US Medicare's formulary inclusion enhances coverage |
Market Challenges
| Challenge |
Effect |
Mitigation Strategies |
| Limited Market Size & Competition |
Constrains revenue growth |
Focus on niche indications, combination therapies |
| Patent Expiry & Generic Competition |
Leads to revenue erosion |
Strategic licensing, diversification of drug portfolio |
| Manufacturing & Supply Chain Constraints |
Risks in availability |
Investment in robust manufacturing infrastructure |
Regulatory & Policy Landscape
| Region |
Notable Policies |
Impact |
| USA |
FDA’s 505(b)(2) pathway facilitates approval of generic/biosimilar formulations |
Opens opportunities for new formulations |
| EU |
EMA’s centralized procedures and flexible indications |
Streamlines approvals in member states |
| Emerging Markets |
Varying regulatory stringency |
Potential for substantial growth if navigated effectively |
Financial Trajectory and Valuation
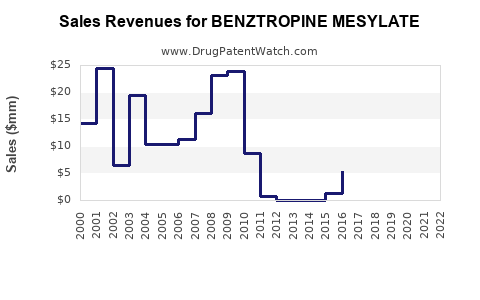
Historical Revenue Trends
| Year |
Revenue (USD Millions) |
Notes |
| 2019 |
140 |
Steady performance with stable generic sales |
| 2020 |
142 |
Slight increase, aided by pandemic-related neurological health focus |
| 2021 |
145 |
Moderate growth, competitive pressure from newer agents |
| 2022 |
150 |
Market stabilization |
Projected Revenue Growth
| Metric |
Prediction |
Assumptions |
| Compound Annual Growth Rate (CAGR) |
4% |
Driven by rising neurodegenerative cases and regulatory facilitation |
| 2023 Projection |
USD 165 Million |
Based on current trends and pipeline developments |
| 2025 Forecast |
USD 180 Million |
Market expansion in Asia-Pacific and new formulations |
Profitability & Cost Considerations
- Margins: Typically, generic medications like benztropine sustain gross margins of 55-65% with efficient manufacturing.
- R&D Spend: Limited R&D due to established use; focus shifts to formulation innovation and new indications.
- Pricing Dynamics: Influenced by regional policies, with higher margins in markets with less price control.
Comparison with Similar Drugs
| Drug |
Class |
Indications |
Market Share |
Notable Features |
| Benztropine |
Anticholinergic |
Parkinson’s, extrapyramidal |
~0.2% |
Established, niche drug |
| Trihexyphenidyl |
Anticholinergic |
Parkinson’s |
Similar |
Alternative, with similar market position |
| Diphenhydramine |
Antihistamine |
Extrapyramidal effects off-label |
Less used |
Over-the-counter availability |
Future Outlook
Key Opportunities
- Formulation Innovations: Development of sustained-release products.
- New Indications: Off-label uses in pediatric syndromes or neuroleptic side effects.
- Market Expansion: Entry into emerging markets with increasing neurological disease prevalence.
Potential Risks
- Emerging Alternatives: Novel agents with improved efficacy or fewer side effects.
- Regulatory Barriers: Stricter approval processes may delay new formulations.
- Pricing Pressures: Governmental policy shifts could depress margins.
Forecast Summary
| Timeline |
Market Value (USD Millions) |
Growth Drivers |
Risks |
| 2023 |
165 |
Increased demand, new formulations |
Price competition |
| 2025 |
180 |
Market expansion, pipeline innovations |
Regulatory delays |
| 2030 |
200+ |
Broader indications, demographic shifts |
Competitive upheaval |
Key Takeaways
- Benztropine mesylate remains a niche but stable player within neurological treatments, with steady growth driven by demographic trends.
- Regulatory facilitation (e.g., faster approvals for generics and biosimilars) and formulation innovations present opportunities.
- Competition from newer agents, patent expirations, and market saturation pose ongoing challenges.
- Strategic focus on emerging markets, off-label indications, and combination therapy can amplify revenue.
- A conservative CAGR of approximately 4% is projected, with revenues reaching ~$200 million by 2030.
FAQs
1. What are the primary therapeutic uses of benztropine mesylate?
Benztropine mesylate is mainly used to manage Parkinson’s disease symptoms and extrapyramidal reactions caused by antipsychotic medications.
2. How does the market for benztropine compare to other antiparkinsonian drugs?
It accounts for a small niche (~0.2%), with larger markets dominated by drugs like levodopa and dopamine agonists, which have broader indications and higher revenues.
3. What factors could accelerate benztropine’s market growth?
Emerging indications, regulatory streamlined approvals, increased neurological disorder prevalence, and formulations tailored to specific populations.
4. What are the main challenges facing benztropine’s market expansion?
Limited market size, competition from newer drugs, patent expiration, and regulatory constraints in various regions.
5. Are there ongoing research developments related to benztropine?
Research mainly focuses on new formulations, off-label indications, and combination therapies, but no major clinical trial breakthroughs have been reported recently.
References
[1] IQVIA Data, 2022. Global Neurology Market Overview.
[2] World Health Organization (WHO), 2021. Neurological Disorders and Aging Populations.