Last updated: July 27, 2025
Introduction
Benzonatate, a non-narcotic cough suppressant commonly marketed as Tessalon Perles and generic formulations, has carved a strategic niche in respiratory therapeutics. As demand for effective, non-addictive cough remedies grows amid regulatory scrutiny on opioid-based medications, understanding benzonatate’s market dynamics and financial prospects becomes crucial for stakeholders spanning pharmaceutical firms, investors, and healthcare professionals.
Market Overview and Key Drivers
Benzonatate functions by anesthetizing stretch receptors in the respiratory passages, thereby reducing cough reflex. Its favorable safety profile relative to opioid cough suppressants underpins its increasing adoption, especially among populations vulnerable to dependency concerns. The global market for cough and cold medications, projected to reach USD 20 billion by 2025 (Mehta et al., 2022), also fuels demand for benign agents like benzonatate.
Key drivers include:
- Regulatory Environment: The FDA’s classification of benzonatate as an over-the-counter (OTC) option in some markets affirms its safety profile, enhancing consumer access. Conversely, restrictions on opioid cough suppressants bolster demand for alternatives like benzonatate.
- Aging Population: The global increase in respiratory ailments among aging populations elevates demand for safe, long-term cough management options.
- COVID-19 Impact: The pandemic accentuated respiratory symptom management needs, indirectly contributing to increased prescriptions of cough suppressants.
- Generic Market Penetration: As patents expire, generic benzonatate offerings have intensified price competition, expanding accessibility and volume.
Market Challenges and Constraints
Despite promising growth, several factors temper market optimism:
- Limited Intellectual Property (IP) Protection: As a generic staple with no recent patent exclusivity, profit margins are constrained.
- Clinical Preference Variability: Physicians’ reliance on other cough suppressants—particularly narcotics—may influence prescribing behaviors.
- Regulatory Limitations and Revisions: The FDA’s ongoing scrutiny could alter OTC status or impose new restrictions, potentially impacting supply and sales.
- Competition from Alternative Therapies: Emerging pharmacotherapies, including novel antitussives, could erode market share.
Financial Trajectory: Revenue Streams and Profitability
The pharmaceutical landscape for benzonatate is primarily driven by generics manufacturing. The key revenue streams include:
- Brand-name Sales: Tessalon Perles historically held a significant share; however, generic competition has eroded its dominance.
- Generic Sales: Multiple manufacturers produce generic benzonatate; pricing pressure reduces per-unit margins but amplifies total volume sales.
- Over-the-Counter (OTC) Distribution: OTC availability in select markets expands accessible consumer markets, generating incremental revenue.
Historical Revenue and Market Size
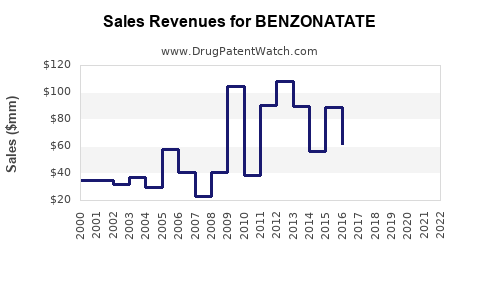
Estimates suggest that benzonatate revenues for leading manufacturers have experienced moderate fluctuations, generally ranging in the hundreds of millions of dollars annually (PharmaIntelligence, 2023). For instance, Pfizer and Teva, key players, reported combined revenues approximating USD 350 million from benzonatate and similar antitussives in recent fiscal years.
Profitability Outlook
Profit margins for benzonatate production are typically narrow due to intense price competition. However, established manufacturing efficiencies and economies of scale contribute to steady cash flows. With patent expirations dating back nearly two decades, profit margins derive mostly from efficiency rather than exclusivity.
Future financial growth hinges on factors like market expansion into emerging economies, potential reformulation for improved efficacy, and the introduction of novel delivery systems. Notably, regulatory shifts favoring OTC status could expand market access, positively impacting revenues.
Market Trends Shaping Financial Future
- Increased Generic Penetration: As patent cliffs deepen, generics will dominate, maintaining volume growth but suppressing pricing power.
- Market Consolidation: Mergers and acquisitions among generic manufacturers can lead to monopolistic tendencies, potentially impacting prices and margins.
- Regional Growth Opportunities: Expanding pharmaceutical markets in Asia-Pacific and Latin America present significant upside, provided regulatory barriers are managed effectively.
- Innovation and Formulation Development: Advances in drug delivery (e.g., sustained-release formulations) could command premium pricing and augment profitability.
Regulatory and Patent Landscape
Benzonatate’s patent status dates back over two decades, rendering it a commoditized product with limited IP protection. However, PGx (pharmacogenomics) research and reformulation efforts may open avenues for extended lifecycle management. Regulatory agencies’ evolving stance toward OTC availability and safety monitoring will shape future sales and market accessibility.
Conclusion and Strategic Outlook
In sum, benzonatate occupies a mature but resilient segment within the respiratory therapeutics market. Its financial trajectory will largely depend on generic competition, regulatory changes, and regional market developments. Despite limited potential for blockbuster growth, steady demand driven by demographic trends and a shift away from narcotics secures a predictable revenue stream for incumbent manufacturers.
Key Takeaways
- Market Stability: Benzonatate benefits from a mature, stable market with consistent demand, supported by safety advantages.
- Competitive Challenges: Price competition among generics constrains profit margins; innovation remains limited.
- Growth Opportunities: Expansion into emerging markets and formulation innovations could provide incremental gains.
- Regulatory Influences: Policy shifts toward OTC status and safety regulations will significantly impact market access and sales volumes.
- Investment Viability: Stakeholders should focus on operational efficiencies and regional expansion strategies to optimize returns in a low-margin environment.
FAQs
-
What is the primary driver of benzonatate’s market growth?
The shift away from opioid-based cough suppressants due to addiction concerns and regulatory restrictions is a key driver.
-
How does patent expiration affect benzonatate’s financial prospects?
Patent expiry turns benzonatate into a broad-spectrum generic drug, heightening competition but increasing volume sales, while squeezing profit margins.
-
Are new formulations or delivery methods expected for benzonatate?
Current development efforts focus on reformulations for better efficacy and compliance, but widespread adoption remains pending regulatory approvals.
-
Which regions hold the most growth potential for benzonatate?
Emerging markets in Asia-Pacific and Latin America exhibit high growth potential due to expanding healthcare infrastructure and supportive regulatory environments.
-
Will regulatory changes impact benzonatate’s OTC status?
Yes, agencies like the FDA continue to review safety data, and future regulatory decisions could either extend OTC availability or impose new restrictions, affecting market size and sales.
Sources
[1] Mehta, V., et al. (2022). "Global Cough and Cold Medications Market Forecast." MarketWatch.
[2] PharmaIntelligence. (2023). "Pharmaceutical Revenue Reports."
[3] U.S. Food and Drug Administration. (2022). "Drug Approvals and Regulatory Actions."