ANNOVERA Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Annovera, and what generic alternatives are available?
Annovera is a drug marketed by Mayne Pharma and is included in one NDA. There are eight patents protecting this drug.
This drug has fourteen patent family members in fourteen countries.
The generic ingredient in ANNOVERA is ethinyl estradiol; segesterone acetate. There are twenty-six drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the ethinyl estradiol; segesterone acetate profile page.
DrugPatentWatch® Generic Entry Outlook for Annovera
Annovera was eligible for patent challenges on August 10, 2022.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be June 21, 2039. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
Summary for ANNOVERA
| International Patents: | 14 |
| US Patents: | 8 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Clinical Trials: | 2 |
| Formulation / Manufacturing: | see details |
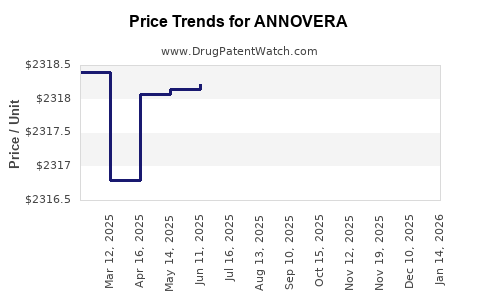
| Drug Prices: | Drug price information for ANNOVERA |
| What excipients (inactive ingredients) are in ANNOVERA? | ANNOVERA excipients list |
| DailyMed Link: | ANNOVERA at DailyMed |
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for ANNOVERA
Generic Entry Date for ANNOVERA*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
RING;VAGINAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for ANNOVERA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| TherapeuticsMD | Phase 1 |
Pharmacology for ANNOVERA
| Drug Class | Estrogen Progestin |
| Mechanism of Action | Estrogen Receptor Agonists |
Anatomical Therapeutic Chemical (ATC) Classes for ANNOVERA
US Patents and Regulatory Information for ANNOVERA
ANNOVERA is protected by eleven US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of ANNOVERA is ⤷ Try a Trial.
This potential generic entry date is based on patent ⤷ Try a Trial.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting ANNOVERA
Method of providing birth control
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF CONTRACEPTION BY INSERTING A VAGINAL SYSTEM FOR UP TO 13 21/7-DAY (IN/OUT) CYCLES, WHEREIN EFFICACY REQUIRES THE SYSTEM CANNOT BE OUT OF THE VAGINA FOR MORE THAN 2 CUMULATIVE HOURS IN ANY SUCH CYCLE WITHOUT USING ALTERNATIVE CONTRACEPTION
Method of providing birth control
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF PREVENTING PREGNANCY BY INSERTING A VAGINAL SYSTEM CONTAINING 103 MG OF SEGESTERONE ACETATE AND 17.4 MG ETHINYL ESTRADIOL INTO A VAGINA FOR UP TO THIRTEEN 21/7-DAY (IN/OUT) CYCLES
Method of providing birth control
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF PREVENTING PREGNANCY BY INSERTING A VAGINAL SYSTEM CONTAINING 103 MG OF SEGESTERONE ACETATE AND 17.4 MG ETHINYL ESTRADIOL INTO A VAGINA FOR UP TO THIRTEEN 21/7-DAY (IN/OUT) CYCLES
Method of providing birth control
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF CONTRACEPTION BY INSERTING A VAGINAL SYSTEM FOR UP TO 13 21/7-DAY (IN/OUT) CYCLES, WHEREIN EFFICACY REQUIRES THE SYSTEM CANNOT BE OUT OF THE VAGINA FOR MORE THAN 2 CUMULATIVE HOURS IN ANY SUCH CYCLE WITHOUT USING ALTERNATIVE CONTRACEPTION
Method of providing birth control
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF CONTRACEPTION BY INSERTING A VAGINAL SYSTEM FOR UP TO 13 21/7-DAY (IN/OUT) CYCLES, WHEREIN EFFICACY REQUIRES THE SYSTEM CANNOT BE OUT OF THE VAGINA FOR MORE THAN 2 CUMULATIVE HOURS IN ANY SUCH CYCLE WITHOUT USING ALTERNATIVE CONTRACEPTION
Method of providing birth control
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF PREVENTING PREGNANCY BY INSERTING A VAGINAL SYSTEM CONTAINING 103 MG OF SEGESTERONE ACETATE AND 17.4 MG ETHINYL ESTRADIOL INTO A VAGINA FOR UP TO THIRTEEN 21/7-DAY (IN/OUT) CYCLES
System for providing birth control
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
System for providing birth control
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
System for providing birth control
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF PREVENTING PREGNANCY BY INSERTING A VAGINAL SYSTEM CONTAINING 103 MG OF SEGESTERONE ACETATE AND 17.4 MG OF ETHINYL ESTRADIOL FOR UP TO 1THIRTEEN 21/7-DAY (IN/OUT) CYCLES
International Patents for ANNOVERA
When does loss-of-exclusivity occur for ANNOVERA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 0976
Estimated Expiration: ⤷ Try a Trial
Australia
Patent: 20294780
Estimated Expiration: ⤷ Try a Trial
Brazil
Patent: 2021025853
Estimated Expiration: ⤷ Try a Trial
Canada
Patent: 41077
Estimated Expiration: ⤷ Try a Trial
Chile
Patent: 21003410
Estimated Expiration: ⤷ Try a Trial
China
Patent: 4364369
Estimated Expiration: ⤷ Try a Trial
Colombia
Patent: 21017509
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 86375
Estimated Expiration: ⤷ Try a Trial
Israel
Patent: 9135
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 22536836
Estimated Expiration: ⤷ Try a Trial
Mexico
Patent: 22000047
Estimated Expiration: ⤷ Try a Trial
South Africa
Patent: 2110288
Estimated Expiration: ⤷ Try a Trial
South Korea
Patent: 220027979
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering ANNOVERA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Argentina | 120976 | SISTEMA PARA PROPORCIONAR UN CONTROL DE LA NATALIDAD | ⤷ Try a Trial |
| Mexico | 2022000047 | SISTEMA PARA PROPORCIONAR CONTROL DE LA NATALIDAD. (SYSTEM FOR PROVIDING BIRTH CONTROL.) | ⤷ Try a Trial |
| South Africa | 202110288 | SYSTEM FOR PROVIDING BIRTH CONTROL | ⤷ Try a Trial |
| China | 114364369 | 用于提供节育的系统 (System for providing birth control) | ⤷ Try a Trial |
| Chile | 2021003410 | Sistema para proporcionar control de la natalidad | ⤷ Try a Trial |
| South Korea | 20220027979 | 산아 제한을 제공하기 위한 시스템 | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for ANNOVERA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0136011 | 2000C/027 | Belgium | ⤷ Try a Trial | PRODUCT NAME: ETHINYLESTRADIOLUM / NORETHISTERONI ACETAS; NAT. REGISTRATION NO/DATE: 19 IS 106 F3 20000911; FIRST REGISTRATION: NL RVG 23909 19991124 |
| 1214076 | SZ 49/2008 | Austria | ⤷ Try a Trial | PRODUCT NAME: WIRKSTOFFKOMBINATION VON ETHINYLESTRADIOL UND DROSPIRENON |
| 1380301 | 2009C/007 | Belgium | ⤷ Try a Trial | PRODUCT NAME: DROSPIRENONE-ETHINYLESTRADIOL; AUTHORISATION NUMBER AND DATE: BE321386 20080811 |
| 1453521 | 15C0050 | France | ⤷ Try a Trial | PRODUCT NAME: ETHINYLESTRADIOL ET MELANGE DE LEVONORGESTREL ET ETHINYLESTRADIOL; NAT. REGISTRATION NO/DATE: NL 42237 20150320; FIRST REGISTRATION: SK - 17/0017/15-S 20150129 |
| 0771217 | CA 2006 00038 | Denmark | ⤷ Try a Trial | PRODUCT NAME: ETHINYLESTRADIOL (SOM BETA-CYCLODEXTRIN-CLATHRAT) OG DROSPIRENON; NAT. REG. NO/DATE: 38687 20060627; FIRST REG. NO/DATE: EU RVG 31781 20050804 |
| 1453521 | 122015000093 | Germany | ⤷ Try a Trial | PRODUCT NAME: LEVONORGESTREL UND ETHINYLESTRADIOL; NAT. REGISTRATION NO/DATE: 87675.00.00 20150720; FIRST REGISTRATION: SLOWAKEI 17/0017/15-S 20150129 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


