ANJESO Drug Patent Profile
✉ Email this page to a colleague
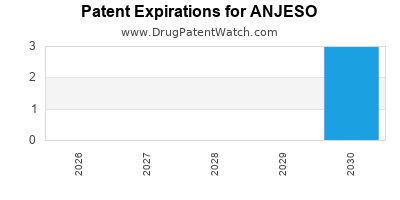
When do Anjeso patents expire, and when can generic versions of Anjeso launch?
Anjeso is a drug marketed by Baudax and is included in one NDA. There are seven patents protecting this drug.
This drug has forty-seven patent family members in thirteen countries.
The generic ingredient in ANJESO is meloxicam. There are twenty-two drug master file entries for this compound. Forty-seven suppliers are listed for this compound. Additional details are available on the meloxicam profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Anjeso
A generic version of ANJESO was approved as meloxicam by AVONDALE PHARMS on June 1st, 2004.
Summary for ANJESO
| International Patents: | 47 |
| US Patents: | 7 |
| Applicants: | 1 |
| NDAs: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 145 |
| Patent Applications: | 3,684 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for ANJESO |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for ANJESO |
| What excipients (inactive ingredients) are in ANJESO? | ANJESO excipients list |
| DailyMed Link: | ANJESO at DailyMed |

Anatomical Therapeutic Chemical (ATC) Classes for ANJESO
US Patents and Regulatory Information for ANJESO
ANJESO is protected by seven US patents.
Patents protecting ANJESO
Nanoparticulate meloxicam formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: MANAGEMENT OF MODERATE-TO-SEVERE PAIN BY INTRAVENOUS INJECTION
Nanoparticulate meloxicam formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: MANAGEMENT OF MODERATE-TO-SEVERE PAIN BY INTRAVENOUS INJECTION
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: MANAGEMENT OF MODERATE-TO-SEVERE PAIN BY INTRAVENOUS INJECTION
Method of treating pain in elderly patients with mild renal impairment
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: MANAGEMENT OF MODERATE-TO-SEVERE PAIN BY INTRAVENOUS INJECTION IN PATIENTS WITH MILD RENAL IMPAIRMENT
Reduction of flake-like aggregation in nanoparticulate active agent compositions
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: MANAGEMENT OF MODERATE-TO-SEVERE PAIN BY INJECTION
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: MANAGEMENT OF MODERATE-TO-SEVERE PAIN BY INJECTION
Reduction of flake-like aggregation in nanoparticulate active agent compositions
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baudax | ANJESO | meloxicam | SOLUTION;INTRAVENOUS | 210583-001 | Feb 20, 2020 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Baudax | ANJESO | meloxicam | SOLUTION;INTRAVENOUS | 210583-001 | Feb 20, 2020 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Baudax | ANJESO | meloxicam | SOLUTION;INTRAVENOUS | 210583-001 | Feb 20, 2020 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Baudax | ANJESO | meloxicam | SOLUTION;INTRAVENOUS | 210583-001 | Feb 20, 2020 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Baudax | ANJESO | meloxicam | SOLUTION;INTRAVENOUS | 210583-001 | Feb 20, 2020 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Baudax | ANJESO | meloxicam | SOLUTION;INTRAVENOUS | 210583-001 | Feb 20, 2020 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for ANJESO
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Baudax | ANJESO | meloxicam | SOLUTION;INTRAVENOUS | 210583-001 | Feb 20, 2020 | ⤷ Try a Trial | ⤷ Try a Trial |
| Baudax | ANJESO | meloxicam | SOLUTION;INTRAVENOUS | 210583-001 | Feb 20, 2020 | ⤷ Try a Trial | ⤷ Try a Trial |
| Baudax | ANJESO | meloxicam | SOLUTION;INTRAVENOUS | 210583-001 | Feb 20, 2020 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for ANJESO
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Norbrook Laboratories (Ireland) Limited | Loxicom | meloxicam | EMEA/V/C/000141 DogsAlleviation of inflammation and pain in both acute and chronic musculoskeletal disorders. To reduce postoperative pain and inflammation following orthopaedic and soft-tissue surgery.CatsAlleviation of inflammation and pain in chronic musculoskeletal disorders in cats. To reduce postoperative pain after ovariohysterectomy and minor soft-tissue surgery.CattleFor use in acute respiratory infection with appropriate antibiotic therapy to reduce clinical signs in cattle. For use in diarrhoea in combination with oral rehydration therapy to reduce clinical signs in calves of over one week of age and young, non-lactating cattle. For adjunctive therapy in the treatment of acute mastitis, in combination with antibiotic therapy.PigsFor use in noninfectious locomotor disorders to reduce the symptoms of lameness and inflammation. For adjunctive therapy in the treatment of puerperal septicaemia and toxaemia (mastitis-metritis-agalactia syndrome) with appropriate antibiotic therapy.HorsesFor use in the alleviation of inflammation and relief of pain in both acute and chronic musculoskeletal disorders.For the relief of pain associated with equine colic. |
Authorised | yes | no | no | 2009-02-10 | |
| Le Vet Beheer B.V. | Novaquin | meloxicam | EMEA/V/C/003866 Alleviation of inflammation and relief of pain in both acute and chronic musculo-skeletal disorders in horses. |
Authorised | no | no | no | 2015-09-08 | |
| Le Vet Beheer B.V | Meloxidolor | meloxicam | EMEA/V/C/002590 DogsAlleviation of inflammation and pain in both acute and chronic musculoskeletal disorders.Reduction of postoperative pain and inflammation following orthopaedic and soft-tissue surgery.CatsReduction of postoperative pain after ovariohysterectomy and minor soft-tissue surgery.CattleFor use in acute respiratory infection with appropriate antibiotic therapy to reduce clinical signs.For use in diarrhoea in combination with oral rehydration therapy to reduce clinical signs in calves of over one week of age and young, non-lactating cattle.For adjunctive therapy in the treatment of acute mastitis, in combination with antibiotic therapy.PigsFor use in noninfectious locomotor disorders to reduce the symptoms of lameness and inflammation.For the relief of postoperative pain associated with minor soft-tissue surgery such as castration.For adjunctive therapy in the treatment of puerperal septicaemia and toxaemia (mastitis-metritis-agalactia syndrome) with appropriate antibiotic therapy.HorsesFor use in the alleviation of inflammation and relief of pain in both acute and chronic musculoskeletal disorders.For the relief of pain associated with equine colic. |
Authorised | yes | no | no | 2013-04-22 | |
| Boehringer Ingelheim Vetmedica GmbH | Novem | meloxicam | EMEA/V/C/000086 Novem 5-mg/ml solution for injection for cattle and pigs:CattleFor use in acute respiratory infection with appropriate antibiotic therapy to reduce clinical signs in cattle.For use in diarrhoea in combination with oral rehydration therapy to reduce clinical signs in calves of over one week of age and young, non-lactating cattle.For the relief of postoperative pain following dehorning in calves.PigsFor use in noninfectious locomotor disorders to reduce the symptoms of lameness and inflammation.For the relief of postoperative pain associated with minor soft-tissue surgery such as castration.Novem 20-mg/ml solution for injection for cattle and pigs:CattleFor use in acute respiratory infection with appropriate antibiotic therapy to reduce clinical signs in cattle.For use in diarrhoea in combination with oral rehydration therapy to reduce clinical signs in calves of over one week of age and young, non-lactating cattle.For adjunctive therapy in the treatment of acute mastitis, in combination with antibiotic therapy.For the relief of postoperative pain following dehorning in calves.PigsFor use in noninfectious locomotor disorders to reduce the symptoms of lameness and inflammation.For adjunctive therapy in the treatment of puerperal septicaemia and toxaemia (mastitis-metritis-agalactia syndrome) with appropriate antibiotic therapy.Novem 40 mg/ml solution for injection for cattle:For use in acute respiratory infection with appropriate antibiotic therapy to reduce clinical signs in cattle.For use in diarrhoea in combination with oral re-hydration therapy to reduce clinical signs in calves of over one week of age and young, non-lactating cattle.For adjunctive therapy in the treatment of acute mastitis, in combination with antibiotic therapy. |
Authorised | no | no | no | 2004-03-02 | |
| Boehringer Ingelheim Vetmedica GmbH | Metacam | meloxicam | EMEA/V/C/000033 Cats:Alleviation of mild to moderate post-operative pain and inflammation following surgical procedures, e.g. orthopaedic and soft tissue surgery.Alleviation of pain and inflammation in acute and chronic musculo-skeletal disorders.Reduction of post-operative pain after ovariohysterectomy and minor soft tissue surgery.Cattle:For use in acute respiratory infection with appropriate antibiotic therapy to reduce clinical signs.For use in diarrhoea in combination with oral re-hydration therapy to reduce clinical signs in calves of over one week of age and young, non-lactating cattle.For the relief of post-operative pain following dehorning in calves.For adjunctive therapy in the treatment of acute mastitis, in combination with antibiotic therapy.Dogs:Alleviation of inflammation and pain in both acute and chronic musculo-skeletal disorders.Reduction of post-operative pain and inflammation following orthopaedic and soft tissue surgery.Horses:For use in the alleviation of inflammation and relief of pain in both acute and chronic musculo-skeletal disorders.For the relief of pain associated with equine colic.Alleviation of inflammation and relief of pain in both acute and chronic musculo-skeletal disorders.Pigs: For use in non-infectious locomotor disorders to reduce the symptoms of lameness and inflammation.For the relief of post-operative pain associated with minor soft tissue surgery such as castration.For adjunctive therapy in the treatment of puerperal septicaemia and toxaemia (mastitis-metritis-agalactia syndrome) with appropriate antibiotic therapy.Guinea pigs:Alleviation of mild to moderate post-operative pain associated with soft tissues surgery such as male castration. |
Authorised | no | no | no | 1998-01-07 | |
| Chanelle Pharmaceuticals Manufacturing Limited | Rheumocam | meloxicam | EMEA/V/C/000121 DogsAlleviation of inflammation and pain in both acute and chronic musculo-skeletal disorders in dogs. To reduce post-operative pain and inflammation following orthopaedic and soft tissue surgery.CatsReduction of post-operative pain after ovariohysterectomy and minor soft tissue surgery.Alleviation of mild to moderate post-operative pain and inflammation following surgical procedures in cats, e.g. orthopaedic and soft tissue surgery.Alleviation of pain and inflammation in acute and chronic musculo-skeletal disorders in cats.CattleFor use in acute respiratory infection with appropriate antibiotic therapy to reduce clinical signs. For use in diarrhoea in combination with oral re-hydration therapy to reduce clinical signs in calves of over one week of age and young, non-lactating cattle. For adjunctive therapy in the treatment of acute mastitis, in combination with antibiotic therapy.PigsFor use in non-infectious locomotor disorders to reduce the symptoms of lameness and inflammation. For adjunctive therapy in the treatment of puerperal septicaemia and toxaemia (mastitis-metritis-agalactia syndrome) with appropriate antibiotic therapy. For the relief of post operative pain associated with minor soft tissue such as castration.HorsesAlleviation of inflammation and relief of pain in both acute and chronic musculo-skeletal disorders in horses..For the relief of pain associated with equine colic. |
Authorised | yes | no | no | 2008-01-10 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for ANJESO
See the table below for patents covering ANJESO around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| European Patent Office | 3167875 | RÉDUCTION D'AGRÉGATION DE TYPE PAILLETTES DANS DES COMPOSITIONS DE MELOXICAM NANOPARTICULAIRE (REDUCTION OF FLAKE-LIKE AGGREGATION IN NANOPARTICULATE MELOXICAM COMPOSITIONS) | ⤷ Try a Trial |
| European Patent Office | 3761977 | MÉTHODES D'ADMINISTRATION DE MELOXICAM PAR VOIE INTRAVEINEUSE EN UNE DOSE BOLUS (METHODS OF ADMINISTERING INTRAVENOUS MELOXICAM IN A BOLUS DOSE) | ⤷ Try a Trial |
| Japan | 2021527697 | ボーラス用量での経静脈メロキシカムの投与方法 | ⤷ Try a Trial |
| South Korea | 20200130334 | 멜록시캄의 일시 투여량을 정맥내 투여하는 방법 | ⤷ Try a Trial |
| Australia | 2019231858 | Methods of administering intravenous meloxicam in a bolus dose | ⤷ Try a Trial |
| Canada | 3117209 | METHODES D'ADMINISTRATION DE MELOXICAM PAR VOIE INTRAVEINEUSE DE MANIERE PREOPERATOIRE ET EN ASSOCIATION AVEC D'AUTRES MEDICAMENTS (METHODS OF ADMINISTERING INTRAVENOUS MELOXICAM PRE-OPERATIVELY AND IN COMBINATION WITH OTHER DRUGS) | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


