AMITIZA Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Amitiza, and what generic alternatives are available?
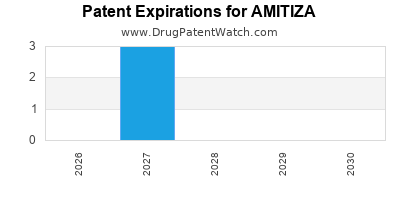
Amitiza is a drug marketed by Sucampo Pharma Llc and is included in one NDA. There are five patents protecting this drug and one Paragraph IV challenge.
This drug has seventy-two patent family members in nineteen countries.
The generic ingredient in AMITIZA is lubiprostone. There are ten drug master file entries for this compound. Sixteen suppliers are listed for this compound. Additional details are available on the lubiprostone profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Amitiza
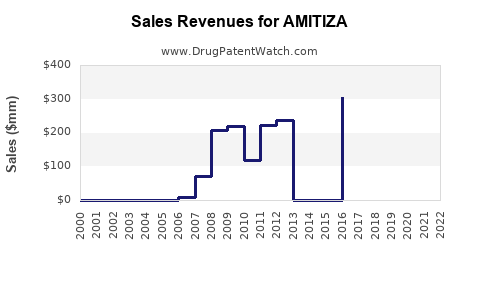
A generic version of AMITIZA was approved as lubiprostone by AMNEAL on November 30th, 2021.
Summary for AMITIZA
| International Patents: | 72 |
| US Patents: | 5 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 10 |
| Raw Ingredient (Bulk) Api Vendors: | 87 |
| Clinical Trials: | 28 |
| Patent Applications: | 1,409 |
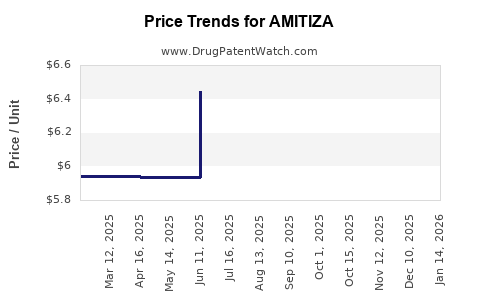
| Drug Prices: | Drug price information for AMITIZA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for AMITIZA |
| What excipients (inactive ingredients) are in AMITIZA? | AMITIZA excipients list |
| DailyMed Link: | AMITIZA at DailyMed |
Recent Clinical Trials for AMITIZA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Sucampo Pharma Americas, LLC | Phase 2 |
| Sucampo AG | Phase 2 |
| Takeda | Phase 2 |
Pharmacology for AMITIZA
| Drug Class | Chloride Channel Activator |
| Mechanism of Action | Chloride Channel Activators |
Anatomical Therapeutic Chemical (ATC) Classes for AMITIZA
Paragraph IV (Patent) Challenges for AMITIZA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| AMITIZA | Capsules | lubiprostone | 8 mcg and 24 mcg | 021908 | 1 | 2012-08-20 |
US Patents and Regulatory Information for AMITIZA
AMITIZA is protected by six US patents.
Patents protecting AMITIZA
Method for treating abdominal discomfort
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: METHOD FOR TREATING IRRITABLE BOWEL SYNDROME AND METHOD FOR TREATING ABDOMINAL DISCOMFORT ASSOCIATED WITH IRRITABLE BOWEL SYNDROME
Soft-gelatin capsule formulation
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Soft-gelatin capsule formulation
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Method for treating gastrointestinal disorder
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: METHOD FOR THE LONG TERM TREATMENT OF CHRONIC CONSTIPATION IN A HUMAN SUBJECT WITH IRRITABLE BOWEL SYNDROME
Method for treating gastrointestinal disorder
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: METHOD FOR THE LONG TERM TREATMENT OF CHRONIC CONSTIPATION IN A HUMAN SUBJECT
Soft-gelatin capsule formulation
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sucampo Pharma Llc | AMITIZA | lubiprostone | CAPSULE;ORAL | 021908-002 | Apr 29, 2008 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Sucampo Pharma Llc | AMITIZA | lubiprostone | CAPSULE;ORAL | 021908-001 | Jan 31, 2006 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | ||
| Sucampo Pharma Llc | AMITIZA | lubiprostone | CAPSULE;ORAL | 021908-002 | Apr 29, 2008 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | ||
| Sucampo Pharma Llc | AMITIZA | lubiprostone | CAPSULE;ORAL | 021908-001 | Jan 31, 2006 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for AMITIZA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Sucampo Pharma Llc | AMITIZA | lubiprostone | CAPSULE;ORAL | 021908-001 | Jan 31, 2006 | ⤷ Sign Up | ⤷ Sign Up |
| Sucampo Pharma Llc | AMITIZA | lubiprostone | CAPSULE;ORAL | 021908-001 | Jan 31, 2006 | ⤷ Sign Up | ⤷ Sign Up |
| Sucampo Pharma Llc | AMITIZA | lubiprostone | CAPSULE;ORAL | 021908-002 | Apr 29, 2008 | ⤷ Sign Up | ⤷ Sign Up |
| Sucampo Pharma Llc | AMITIZA | lubiprostone | CAPSULE;ORAL | 021908-002 | Apr 29, 2008 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for AMITIZA
When does loss-of-exclusivity occur for AMITIZA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 07208632
Estimated Expiration: ⤷ Sign Up
Brazil
Patent: 0707334
Estimated Expiration: ⤷ Sign Up
Canada
Patent: 37274
Estimated Expiration: ⤷ Sign Up
China
Patent: 1410097
Estimated Expiration: ⤷ Sign Up
Patent: 4971056
Estimated Expiration: ⤷ Sign Up
Patent: 4983712
Estimated Expiration: ⤷ Sign Up
Denmark
Patent: 78944
Estimated Expiration: ⤷ Sign Up
European Patent Office
Patent: 78944
Estimated Expiration: ⤷ Sign Up
Israel
Patent: 2867
Estimated Expiration: ⤷ Sign Up
Japan
Patent: 83794
Estimated Expiration: ⤷ Sign Up
Patent: 08534432
Estimated Expiration: ⤷ Sign Up
Mexico
Patent: 08009650
Estimated Expiration: ⤷ Sign Up
New Zealand
Patent: 0191
Estimated Expiration: ⤷ Sign Up
Portugal
Patent: 78944
Estimated Expiration: ⤷ Sign Up
Russian Federation
Patent: 20291
Estimated Expiration: ⤷ Sign Up
Patent: 08134489
Estimated Expiration: ⤷ Sign Up
South Korea
Patent: 1393944
Estimated Expiration: ⤷ Sign Up
Patent: 080090526
Estimated Expiration: ⤷ Sign Up
Spain
Patent: 92590
Estimated Expiration: ⤷ Sign Up
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering AMITIZA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| European Patent Office | 2298314 | Unité posologique contenant un analogue de prostaglandine permettant de traiter la constipation (Dosage unit comprising a prostaglandin analog for treating constipation) | ⤷ Sign Up |
| Mexico | PA04002006 | ANALOGOS DE PROSTAGLADINA COMO ABRIDOR DEL CANAL DE CLORURO. (PROSTAGLANDIN ANALOGS AS CHLORIDE CHANNEL OPENER.) | ⤷ Sign Up |
| Japan | 2004527567 | ⤷ Sign Up | |
| Spain | 2309410 | ⤷ Sign Up | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for AMITIZA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1315485 | 300757 | Netherlands | ⤷ Sign Up | PRODUCT NAME: LUBIPROSTON; NATIONAL REGISTRATION NO/DATE: RVG 115891; FIRST REGISTRATION NO/DATE: PL21341/0003 20120910 |
| 2298314 | 92826 | Luxembourg | ⤷ Sign Up | PRODUCT NAME: LUBIPROSTONE, INCLUANT LES SELS PHARMACEUTIQUEMENT ACCEPTABLES, LES ESTERS OU LES AMIDES DE LA LUBIPROSTONE. FIRST REGISTRATION: 20120910 |
| 1315485 | C01315485/01 | Switzerland | ⤷ Sign Up | FORMER REPRESENTATIVE: BOHEST AG, CH |
| 1315485 | 122015000080 | Germany | ⤷ Sign Up | PRODUCT NAME: LUBIPROSTON, ODER EIN PHARMAZEUTISCH ANNEHMBARES SALZ DAVON; NAT. REGISTRATION NO/DATE: 92699.00.00 20150519; FIRST REGISTRATION: VEREINIGTES KOENIGREICH PL 21341/0003 20120910 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


