ALECENSA Drug Patent Profile
✉ Email this page to a colleague
When do Alecensa patents expire, and when can generic versions of Alecensa launch?
Alecensa is a drug marketed by Hoffmann-la Roche and is included in one NDA. There are five patents protecting this drug and one Paragraph IV challenge.
This drug has one hundred and thirty-six patent family members in thirty-nine countries.
The generic ingredient in ALECENSA is alectinib hydrochloride. One supplier is listed for this compound. Additional details are available on the alectinib hydrochloride profile page.
DrugPatentWatch® Generic Entry Outlook for Alecensa
Alecensa was eligible for patent challenges on December 11, 2019.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be April 24, 2035. This may change due to patent challenges or generic licensing.
There is one Paragraph IV patent challenge for this drug. This may lead to patent invalidation or a license for generic production.
Indicators of Generic Entry
Summary for ALECENSA
| International Patents: | 136 |
| US Patents: | 5 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 49 |
| Clinical Trials: | 21 |
| Patent Applications: | 46 |
| Drug Prices: | Drug price information for ALECENSA |
| What excipients (inactive ingredients) are in ALECENSA? | ALECENSA excipients list |
| DailyMed Link: | ALECENSA at DailyMed |
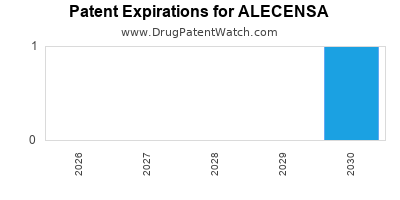

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for ALECENSA
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for ALECENSA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| University of Manchester | Phase 2/Phase 3 |
| Hoffmann-La Roche | Phase 2/Phase 3 |
| University of Birmingham | Phase 2/Phase 3 |
Pharmacology for ALECENSA
| Drug Class | Kinase Inhibitor |
| Mechanism of Action | Kinase Inhibitors |
Anatomical Therapeutic Chemical (ATC) Classes for ALECENSA
Paragraph IV (Patent) Challenges for ALECENSA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| ALECENSA | Capsules | alectinib hydrochloride | 150 mg | 208434 | 1 | 2019-12-11 |
US Patents and Regulatory Information for ALECENSA
ALECENSA is protected by five US patents and one FDA Regulatory Exclusivity.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of ALECENSA is ⤷ Sign Up.
This potential generic entry date is based on patent ⤷ Sign Up.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting ALECENSA
Preparation containing tetracyclic compound at high dose
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Preparation containing tetracyclic compound at high dose
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Tetracyclic compound
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Composition comprising tetracyclic compound
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Tetracyclic compound
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
FDA Regulatory Exclusivity protecting ALECENSA
FOR TREATMENT OF PATIENTS WITH ANAPLASTIC LYMPHOMA KINASE (ALK) POSITIVE, METASTATIC NON-SMALL-CELL LUNG CANCER (NSCLC) AS DETECTED BY AN FDA APPROVED TEST, EXCLUDING PATIENTS WHO HAVE PROGRESSED ON OR ARE INTOLERANT TO CRIZOTINIB
Exclusivity Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hoffmann-la Roche | ALECENSA | alectinib hydrochloride | CAPSULE;ORAL | 208434-001 | Dec 11, 2015 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Hoffmann-la Roche | ALECENSA | alectinib hydrochloride | CAPSULE;ORAL | 208434-001 | Dec 11, 2015 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Hoffmann-la Roche | ALECENSA | alectinib hydrochloride | CAPSULE;ORAL | 208434-001 | Dec 11, 2015 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Hoffmann-la Roche | ALECENSA | alectinib hydrochloride | CAPSULE;ORAL | 208434-001 | Dec 11, 2015 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for ALECENSA
When does loss-of-exclusivity occur for ALECENSA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 0187
Estimated Expiration: ⤷ Sign Up
Australia
Patent: 15250574
Estimated Expiration: ⤷ Sign Up
Patent: 20230293
Estimated Expiration: ⤷ Sign Up
Brazil
Patent: 2016021206
Estimated Expiration: ⤷ Sign Up
Canada
Patent: 46518
Estimated Expiration: ⤷ Sign Up
Patent: 40565
Estimated Expiration: ⤷ Sign Up
China
Patent: 6456651
Estimated Expiration: ⤷ Sign Up
Patent: 3975243
Estimated Expiration: ⤷ Sign Up
European Patent Office
Patent: 35287
Estimated Expiration: ⤷ Sign Up
Israel
Patent: 8363
Estimated Expiration: ⤷ Sign Up
Patent: 3152
Estimated Expiration: ⤷ Sign Up
Japan
Patent: 2015163448
Estimated Expiration: ⤷ Sign Up
Patent: 59712
Estimated Expiration: ⤷ Sign Up
Patent: 29942
Estimated Expiration: ⤷ Sign Up
Patent: 16104762
Estimated Expiration: ⤷ Sign Up
Malaysia
Patent: 9913
Estimated Expiration: ⤷ Sign Up
Mexico
Patent: 16013809
Estimated Expiration: ⤷ Sign Up
Patent: 21012300
Estimated Expiration: ⤷ Sign Up
New Zealand
Patent: 4713
Estimated Expiration: ⤷ Sign Up
Patent: 3604
Estimated Expiration: ⤷ Sign Up
Russian Federation
Patent: 24056
Estimated Expiration: ⤷ Sign Up
Patent: 16145057
Estimated Expiration: ⤷ Sign Up
Patent: 20119391
Estimated Expiration: ⤷ Sign Up
Singapore
Patent: 202009484W
Estimated Expiration: ⤷ Sign Up
Patent: 201607623X
Estimated Expiration: ⤷ Sign Up
South Africa
Patent: 1606447
Estimated Expiration: ⤷ Sign Up
South Korea
Patent: 2412321
Estimated Expiration: ⤷ Sign Up
Patent: 2478887
Estimated Expiration: ⤷ Sign Up
Patent: 160146800
Patent: 4환성 화합물을 고용량 함유하는 제제 (4 Preparation containing tetracyclic compound at high dose)
Estimated Expiration: ⤷ Sign Up
Patent: 220087583
Patent: 4환성 화합물을 고용량 함유하는 제제 (4 Preparation containing tetracyclic compound at high dose)
Estimated Expiration: ⤷ Sign Up
Taiwan
Patent: 20943
Estimated Expiration: ⤷ Sign Up
Patent: 71839
Estimated Expiration: ⤷ Sign Up
Patent: 31128
Estimated Expiration: ⤷ Sign Up
Patent: 1622706
Estimated Expiration: ⤷ Sign Up
Patent: 2114693
Estimated Expiration: ⤷ Sign Up
Patent: 2235088
Estimated Expiration: ⤷ Sign Up
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering ALECENSA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Poland | 2975024 | ⤷ Sign Up | |
| Malaysia | 189913 | PREPARATION CONTAINING TETRACYCLIC COMPOUND AT HIGH DOSE | ⤷ Sign Up |
| Russian Federation | 2020119391 | ПОЛУЧЕНИЕ ТЕТРАЦИКЛИЧЕСКОГО СОЕДИНЕНИЯ, СОДЕРЖАЩЕГОСЯ В ВЫСОКОЙ ДОЗЕ | ⤷ Sign Up |
| Taiwan | 202235088 | Formulation comprising tetracyclic compounds in high dose | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for ALECENSA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2441753 | 2017026 | Norway | ⤷ Sign Up | PRODUCT NAME: ALEKTINIB ELLER SALT ELLER SOLVAT; REG. NO/DATE: EU/1/16/1169 20170307 |
| 2441753 | 31/2017 | Austria | ⤷ Sign Up | PRODUCT NAME: ALECTINIB ODER DESSEN SALZE ODER DESSEN SOLVATE; REGISTRATION NO/DATE: EU/1/16/1169 20170220 |
| 2441753 | SPC/GB17/036 | United Kingdom | ⤷ Sign Up | PRODUCT NAME: ALECTINIB (9-ETHYL-6,6-DIMETHYL-8-(4-MORPHOLIN-4-YL-PIPERIDIN-1-YL)-11-OXO-6,11-DIHYDRO-5H-BENZO(B)CARBAZOLE-3-CARBONITRILE) OR A SALT OR SOLVATE THEREOF; REGISTERED: UK EU/1/16/1169 (NI) 20170220; UK PLGB 00031/0843 20170220 |
| 2441753 | 122017000048 | Germany | ⤷ Sign Up | PRODUCT NAME: ALECTINIB ODER EIN SALZ ODER SOLVAT DAVON; REGISTRATION NO/DATE: EU/1/16/1169 20170216 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


