ABRAXANE Drug Patent Profile
✉ Email this page to a colleague
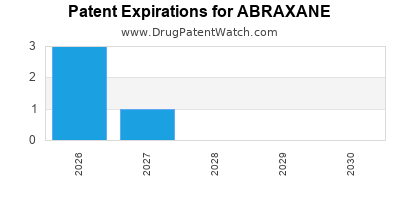
When do Abraxane patents expire, and when can generic versions of Abraxane launch?
Abraxane is a drug marketed by Bristol-myers and is included in one NDA. There are eleven patents protecting this drug and one Paragraph IV challenge.
This drug has two hundred and forty-two patent family members in thirty-one countries.
The generic ingredient in ABRAXANE is paclitaxel. There are sixty-nine drug master file entries for this compound. Eighteen suppliers are listed for this compound. Additional details are available on the paclitaxel profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Abraxane
A generic version of ABRAXANE was approved as paclitaxel by TEVA PHARMS on January 25th, 2002.
Summary for ABRAXANE
| International Patents: | 242 |
| US Patents: | 11 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 3 |
| Raw Ingredient (Bulk) Api Vendors: | 95 |
| Clinical Trials: | 446 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for ABRAXANE |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for ABRAXANE |
| What excipients (inactive ingredients) are in ABRAXANE? | ABRAXANE excipients list |
| DailyMed Link: | ABRAXANE at DailyMed |

Recent Clinical Trials for ABRAXANE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Fujian Medical University Union Hospital | Phase 2 |
| Morten Ladekarl | Phase 2 |
| Herlev and Gentofte Hospital | Phase 2 |
Pharmacology for ABRAXANE
| Drug Class | Microtubule Inhibitor |
| Physiological Effect | Microtubule Inhibition |
Anatomical Therapeutic Chemical (ATC) Classes for ABRAXANE
Paragraph IV (Patent) Challenges for ABRAXANE
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| ABRAXANE | For Injection Suspension | paclitaxel | 100 mg/vial | 021660 | 1 | 2015-12-11 |
US Patents and Regulatory Information for ABRAXANE
ABRAXANE is protected by eleven US patents.
Patents protecting ABRAXANE
Combinations and modes of administration of therapeutic agents and combination therapy
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Compositions and methods of delivery of pharmacological agents
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Compositions and methods of delivery of pharmacological agents
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Combinations and modes of administration of therapeutic agents and combination therapy
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Compositions and methods of delivery of pharmacological agents
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Combinations and modes of administration of therapeutic agents and combination therapy
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Compositions and methods of delivery of pharmacological agents
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Combinations and modes of administration of therapeutic agents and combination therapy
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Methods of treating cancer
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Methods of treating pancreatic cancer
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Methods of treating cancer
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bristol-myers | ABRAXANE | paclitaxel | POWDER;INTRAVENOUS | 021660-001 | Jan 7, 2005 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Bristol-myers | ABRAXANE | paclitaxel | POWDER;INTRAVENOUS | 021660-001 | Jan 7, 2005 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Bristol-myers | ABRAXANE | paclitaxel | POWDER;INTRAVENOUS | 021660-001 | Jan 7, 2005 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Bristol-myers | ABRAXANE | paclitaxel | POWDER;INTRAVENOUS | 021660-001 | Jan 7, 2005 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Bristol-myers | ABRAXANE | paclitaxel | POWDER;INTRAVENOUS | 021660-001 | Jan 7, 2005 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for ABRAXANE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Bristol-myers | ABRAXANE | paclitaxel | POWDER;INTRAVENOUS | 021660-001 | Jan 7, 2005 | ⤷ Try a Trial | ⤷ Try a Trial |
| Bristol-myers | ABRAXANE | paclitaxel | POWDER;INTRAVENOUS | 021660-001 | Jan 7, 2005 | ⤷ Try a Trial | ⤷ Try a Trial |
| Bristol-myers | ABRAXANE | paclitaxel | POWDER;INTRAVENOUS | 021660-001 | Jan 7, 2005 | ⤷ Try a Trial | ⤷ Try a Trial |
| Bristol-myers | ABRAXANE | paclitaxel | POWDER;INTRAVENOUS | 021660-001 | Jan 7, 2005 | ⤷ Try a Trial | ⤷ Try a Trial |
| Bristol-myers | ABRAXANE | paclitaxel | POWDER;INTRAVENOUS | 021660-001 | Jan 7, 2005 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for ABRAXANE
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Bristol-Myers Squibb Pharma EEIG | Abraxane | paclitaxel | EMEA/H/C/000778 Abraxane monotherapy is indicated for the treatment of metastatic breast cancer in adult patients who have failed first-line treatment for metastatic disease and for whom standard, anthracycline containing therapy is not indicated.Abraxane in combination with gemcitabine is indicated for the first-line treatment of adult patients with metastatic adenocarcinoma of the pancreas.Abraxane in combination with carboplatin is indicated for the first-line treatment of non-small cell lung cancer in adult patients who are not candidates for potentially curative surgery and/or radiation therapy. |
Authorised | no | no | no | 2008-01-11 | |
| ratiopharm GmbH | Pazenir | paclitaxel | EMEA/H/C/004441 Pazenir monotherapy is indicated for the treatment of metastatic breast cancer in adult patients who have failed first-line treatment for metastatic disease and for whom standard, anthracycline containing therapy is not indicated.Pazenir in combination with carboplatin is indicated for the first-line treatment of non-small cell lung cancer in adult patients who are not candidates for potentially curative surgery and/or radiation therapy. |
Authorised | yes | no | no | 2019-05-06 | |
| Inceptua AB | Apealea | paclitaxel | EMEA/H/C/004154 Apealea in combination with carboplatin is indicated for the treatment of adult patients with first relapse of platinum‑sensitive epithelial ovarian cancer, primary peritoneal cancer and fallopian tube cancer. |
Authorised | no | no | no | 2018-11-20 | |
| Norton Healthcare Ltd. | Paxene | paclitaxel | EMEA/H/C/000216 Paxene is indicated for the treatment of patients with:• advanced AIDS-related Kaposi's sarcoma (AIDS-KS) who have failed prior liposomal anthracycline therapy;• metastatic carcinoma of the breast (MBC) who have failed, or are not candidates for standard anthracycline-containing therapy;• advanced carcinoma of the ovary (AOC) or with residual disease (> 1 cm) after initial laparotomy, in combination with cisplatin as first-line treatment;• metastatic carcinoma of the ovary (MOC) after failure of platinum-containing combination therapy without taxanes as second-line treatment;• non-small cell lung carcinoma (NSCLC) who are not candidates for potentially curative surgery and/or radiation therapy, in combination with cisplatin. Limited efficacy data supports this indication (see section 5.1). |
Withdrawn | no | no | no | 1999-07-19 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for ABRAXANE
See the table below for patents covering ABRAXANE around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| China | 104587479 | COMPOSITIONS AND METHODS OF DELIVERY OF PHARMACOLOGICAL AGENTS | ⤷ Try a Trial |
| China | 1267214 | ⤷ Try a Trial | |
| Japan | 2020023564 | 組成物及び薬物送達方法 (COMPOSITION AND METHOD OF DELIVERY OF PHARMACOLOGICAL AGENT) | ⤷ Try a Trial |
| Australia | 7117196 | ⤷ Try a Trial | |
| China | 102343094 | Compositions and methods of delivery of pharmacological agents | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for ABRAXANE
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0961612 | 441 | Finland | ⤷ Try a Trial | |
| 1853250 | 2014C/037 | Belgium | ⤷ Try a Trial | PRODUCT NAME: PACLITAXEL DANS UNE FORMULATION DE NANOPARTICULE LIEES A L'ALBUMINE; AUTHORISATION NUMBER AND DATE: EU/1/07/428 20131230 |
| 1853250 | 132014902271575 | Italy | ⤷ Try a Trial | PRODUCT NAME: PACLITAXEL LEGATO ALL'ALBUMINA FORMULATO IN NANOPARTICELLE(ABRAXANE); AUTHORISATION NUMBER(S) AND DATE(S): EU/01/07/428, 20131220 |
| 0961612 | 132009901771196 | Italy | ⤷ Try a Trial | PRODUCT NAME: PACLITAXEL(ABRAXANE); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/07/428/001, 20080114 |
| 0961612 | SZ 41/2009 | Austria | ⤷ Try a Trial | PRODUCT NAME: PACLITAXEL ALBUMIN |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


