Mirtazapine - Generic Drug Details
✉ Email this page to a colleague
What are the generic sources for mirtazapine and what is the scope of patent protection?
Mirtazapine
is the generic ingredient in three branded drugs marketed by Acme Labs, Actavis Elizabeth, Actavis Labs Fl Inc, Aurobindo Pharma, Impax Labs Inc, Organon Usa Organon, Annora Pharma, Apotex, Aurobindo, Chartwell Rx, Ivax Sub Teva Pharms, Mylan, Pharmobedient, Prasco, Roxane, Sun Pharm Inds Inc, Teva, Upsher Smith Labs, Watson Labs, and Organon, and is included in twenty-four NDAs. Additional information is available in the individual branded drug profile pages.There are eighteen drug master file entries for mirtazapine. Thirty-one suppliers are listed for this compound.
Summary for mirtazapine
| US Patents: | 0 |
| Tradenames: | 3 |
| Applicants: | 20 |
| NDAs: | 24 |
| Drug Master File Entries: | 18 |
| Finished Product Suppliers / Packagers: | 31 |
| Raw Ingredient (Bulk) Api Vendors: | 1 |
| Clinical Trials: | 109 |
| Patent Applications: | 7,522 |
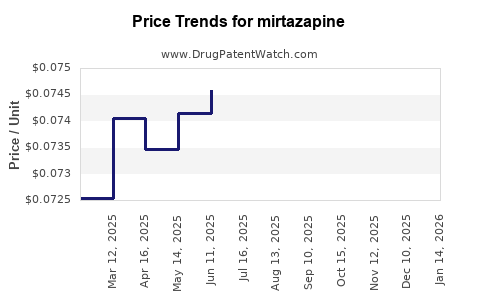
| Drug Prices: | Drug price trends for mirtazapine |
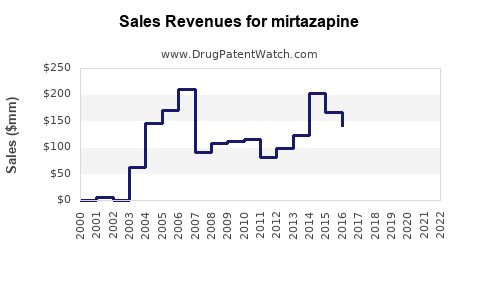
| Drug Sales Revenues: | Drug sales revenues for mirtazapine |
| What excipients (inactive ingredients) are in mirtazapine? | mirtazapine excipients list |
| DailyMed Link: | mirtazapine at DailyMed |
Recent Clinical Trials for mirtazapine
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| University of Oxford | NA |
| Mayo Clinic | EARLY_PHASE1 |
| Washington State University | PHASE2 |
Anatomical Therapeutic Chemical (ATC) Classes for mirtazapine
US Patents and Regulatory Information for mirtazapine
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Organon Usa Organon | REMERON SOLTAB | mirtazapine | TABLET, ORALLY DISINTEGRATING;ORAL | 021208-003 | Jan 12, 2001 | AB | RX | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Organon Usa Organon | REMERON SOLTAB | mirtazapine | TABLET, ORALLY DISINTEGRATING;ORAL | 021208-002 | Jan 12, 2001 | AB | RX | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Chartwell Rx | MIRTAZAPINE | mirtazapine | TABLET;ORAL | 076219-003 | Jun 19, 2003 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Organon Usa Organon | REMERON SOLTAB | mirtazapine | TABLET, ORALLY DISINTEGRATING;ORAL | 021208-001 | Jan 12, 2001 | AB | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Aurobindo | MIRTAZAPINE | mirtazapine | TABLET;ORAL | 076921-002 | Oct 22, 2004 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for mirtazapine
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Organon | REMERON | mirtazapine | TABLET;ORAL | 020415-002 | Jun 14, 1996 | 5,977,099 | ⤷ Get Started Free |
| Organon Usa Organon | REMERON SOLTAB | mirtazapine | TABLET, ORALLY DISINTEGRATING;ORAL | 021208-001 | Jan 12, 2001 | 5,178,878 | ⤷ Get Started Free |
| Organon Usa Organon | REMERON SOLTAB | mirtazapine | TABLET, ORALLY DISINTEGRATING;ORAL | 021208-003 | Jan 12, 2001 | 5,178,878 | ⤷ Get Started Free |
| Organon Usa Organon | REMERON SOLTAB | mirtazapine | TABLET, ORALLY DISINTEGRATING;ORAL | 021208-002 | Jan 12, 2001 | 5,977,099 | ⤷ Get Started Free |
| Organon Usa Organon | REMERON SOLTAB | mirtazapine | TABLET, ORALLY DISINTEGRATING;ORAL | 021208-001 | Jan 12, 2001 | 5,977,099 | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for mirtazapine
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Dechra Regulatory B.V. | Mirataz | mirtazapine | EMEA/V/C/004733For bodyweight gain in cats experiencing poor appetite and weight loss resulting from chronic medical conditions. | Authorised | no | no | no | 2019-12-10 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
Market Dynamics and Financial Trajectory for Mirtazapine
More… ↓