Last updated: July 27, 2025
Introduction
Hydroxychloroquine sulfate (HCQ) is an antimalarial and immunomodulatory medication traditionally used for malaria, lupus erythematosus, and rheumatoid arthritis. Its resurgence in public and private sectors has been notably marked during the COVID-19 pandemic, prompting significant shifts in market dynamics and financial trajectories. This report examines these factors, analyzing current trends, commercial landscape, regulatory considerations, and future projections impacting HCQ.
Market Overview
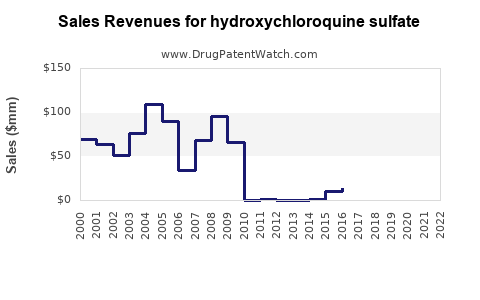
Hydroxychloroquine sulfate has been a fixture in pharmaceutical treatments for decades, primarily recognized for its safety profile and affordability. However, its widespread attention surged during 2020 when it was considered a potential treatment for COVID-19, leading to unparalleled demand spikes. Post this period, its market trajectory has been influenced by regulatory decisions, scientific consensus, and geopolitical factors.
Key Markets:
- North America (notably the U.S.)
- Europe (EU member states)
- Asia-Pacific (including India, China)
- Latin America (e.g., Brazil)
Market Size and Growth:
Prior to the pandemic, the HCQ market size was valued at approximately USD 1.2 billion in 2019, with a steady CAGR of around 4-5%. The COVID-19 period saw an unprecedented demand surge, with some estimates indicating a growth spike exceeding 300% in certain regions during early 2020. However, as scientific consensus shifted and regulatory bodies contraindicated HCQ for COVID-19, the market experienced rapid contraction.
Market Drivers
-
Established Medical Use and Cost-Effectiveness
HCQ remains a frontline therapy for lupus and rheumatoid arthritis, with a well-documented safety profile, strong patent protection, and widespread prescriber familiarity, underpinning stable demand.
-
Emerging Research and Off-Label Uses
Ongoing investigations into HCQ’s immunomodulatory properties furnish potential expansion in autoimmune therapies and other indications, though evidence remains mixed.
-
Global Demand in Low- and Middle-Income Countries (LMICs)
India, as one of the largest producers and consumers, maintains robust demand, driven by affordability and local manufacturing.
-
Pandemic-Related Fluctuations
Initial global panic and stockpiling during early COVID-19 generated demand explosions—targets for both legitimate use and misuse.
Market Constraints
-
Regulatory Setbacks
Major health authorities, notably the U.S. FDA and WHO, withdrew emergency use authorizations and issued safety advisories against HCQ for COVID-19 treatment, significantly dampening demand.
-
Scientific Evidence and Clinical Trials
Large-scale randomized controlled trials (RCTs) failed to demonstrate efficacy for COVID-19, tarnishing HCQ’s reputation and limiting off-label prescriptions.
-
Legal and Ethical Issues
Proliferation of stockpiling, black-market trade, and fraudulent claims have complicated supply chains and regulatory oversight.
-
Patent and Competition Factors
While HCQ's patent has long expired, generic manufacturers dominate, fostering price erosion. Competition from other antimalarial and immunomodulatory drugs limits pricing power.
Regulatory and Legal Landscape
HCQ’s regulatory path is characterized by:
-
Approval Status:
In most jurisdictions, HCQ remains approved for approved indications, with no recent global regulatory restrictions, aside from COVID-related emergency authorizations.
-
Safety Concerns and Labeling:
Regulatory agencies issued warnings to mitigate misuse, highlighting risks of cardiotoxicity and other adverse events, influencing prescriber behavior.
-
Patent Situations:
Patent expiration in the early 2000s led to widespread generics, causing price compression but ensuring continued affordable access.
Financial Trajectory Analysis
Historical Trends:
The 2020 demand spike temporarily elevated revenue and led to shortages, causing manufacturing to scale rapidly. Following scientific disapproval for COVID-19, revenues declined sharply, expected to stabilize at pre-pandemic levels.
Forecasts (2023–2028):
- Moderate growth expected driven by ongoing use in autoimmune diseases.
- The CAGR is projected at 2-3%, reflecting market saturation and limited new indications.
- Potential growth avenues include combination therapies and novel formulations, possibly boosting revenue streams.
Pricing Dynamics:
- Price erosion persists due to generic competition.
- Specialty formulations or combination drugs could command premium pricing in niche segments.
Manufacturing and Supply Chain Considerations:
India and China dominate HCQ production, with capacity expanding in response to pandemic needs. Supply chain resilience remains a strategic concern, especially amid geopolitical tensions and global health crises.
Future Outlook
The HCQ market’s future hinges on several factors:
-
Scientific Validity of New Indications:
Emerging research could revive HCQ’s profile if proven effective in other autoimmune or infectious diseases.
-
Regulatory Environment:
Authorities’ stance on off-label use and safety monitoring will influence market stability.
-
Patent and Market Entry:
Although patents have expired, new formulations or delivery methods could create niche markets.
-
Global Healthcare Trends:
Growing emphasis on personalized medicine and immunomodulatory therapies could reopen interest.
Strategic Market Considerations
-
For Manufacturers:
Investments in quality manufacturing, supply chain diversification, and emerging markets are vital to sustain profitability.
-
For Investors:
Stable core demand in chronic autoimmune indications presents a resilient investment profile, while pandemic-driven fluctuations are unpredictable.
-
For Regulators:
Clear guidelines and safety advisories create a controlled environment, encouraging responsible usage.
Key Takeaways
-
Hydroxychloroquine sulfate’s market experienced rapid growth during COVID-19, driven by emergency use authorizations, but this has largely reversed as scientific evidence contradicts efficacy for COVID-19.
-
The medication’s baseline demand remains steady, fueled by its established use in autoimmune diseases, especially in North America, Europe, and Asia.
-
Patent expiry and generic manufacturing sustain affordability but limit pricing power. Competition from other immunomodulators constrains premium pricing opportunities.
-
Regulatory agencies’ safety warnings shape prescriber behavior, influencing market stability.
-
Future growth potential relies on new clinical evidence, formulation innovations, and strategic positioning in autoimmune and infectious disease therapeutics.
FAQs
1. Will hydroxychloroquine sulfate regain popularity for COVID-19 treatment?
Current scientific consensus indicates limited efficacy for COVID-19, and regulatory agencies have revoked emergency authorizations. Future use may be considered only within clinical trials or for specific high-risk populations under strict oversight.
2. What are the main therapeutic indications sustaining HCQ demand?
Its primary licensed use includes lupus erythematosus and rheumatoid arthritis, where it delivers immunomodulatory benefits with a well-characterized safety profile.
3. How do patent expirations affect the hydroxychloroquine market?
Patent expiration in the early 2000s facilitated generic manufacturing, resulting in price reductions and broader access but decreased revenue margins for brand owners.
4. Are there emerging markets or indications for hydroxychloroquine?
Research into HCQ’s potential in other autoimmune or infectious diseases continues, but no significant new indications are imminent. LMICs, notably India, remain primary markets due to affordability and local manufacturing.
5. What factors could influence the future financial performance of HCQ?
New clinical evidence supporting alternative uses, regulatory changes, formulation innovations, and supply chain developments are key determinants of future revenue trajectories.
References
[1] Market Research Future. (2022). Hydroxychloroquine Sulfate Market Analysis.
[2] Global Data. (2023). Pharmaceuticals: Key Trends and Forecasts.
[3] WHO. (2020). Hydroxychloroquine and COVID-19: Scientific Review.
[4] U.S. FDA. (2020). Safety Communication on Hydroxychloroquine.
[5] Statista. (2022). Global Hydroxychloroquine Market Value and Forecast.