Terbutaline sulfate - Generic Drug Details
✉ Email this page to a colleague
What are the generic drug sources for terbutaline sulfate and what is the scope of freedom to operate?
Terbutaline sulfate
is the generic ingredient in four branded drugs marketed by Novartis, Sanofi Aventis Us, Pharmacare, Chartwell Injectable, Dr Reddys, Epic Pharma Llc, Fresenius Kabi Usa, Hikma Farmaceutica, United Biomedcl, Ani Pharms, Impax Labs, Lannett Co Inc, and Twi Pharms, and is included in fifteen NDAs. Additional information is available in the individual branded drug profile pages.There are three drug master file entries for terbutaline sulfate. Eleven suppliers are listed for this compound.
Summary for terbutaline sulfate
| US Patents: | 0 |
| Tradenames: | 4 |
| Applicants: | 13 |
| NDAs: | 15 |
| Drug Master File Entries: | 3 |
| Finished Product Suppliers / Packagers: | 11 |
| Raw Ingredient (Bulk) Api Vendors: | 71 |
| Clinical Trials: | 3 |
| Patent Applications: | 3,754 |
| Formulation / Manufacturing: | see details |
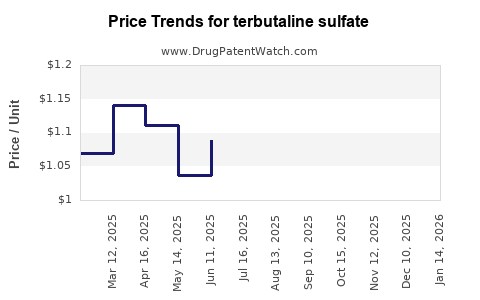
| Drug Prices: | Drug price trends for terbutaline sulfate |
| What excipients (inactive ingredients) are in terbutaline sulfate? | terbutaline sulfate excipients list |
| DailyMed Link: | terbutaline sulfate at DailyMed |
Recent Clinical Trials for terbutaline sulfate
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| The Emmes Company, LLC | Phase 2/Phase 3 |
| Duke Health | Phase 2/Phase 3 |
| Kanecia Zimmerman, MD MPH | Phase 2/Phase 3 |
Medical Subject Heading (MeSH) Categories for terbutaline sulfate
US Patents and Regulatory Information for terbutaline sulfate
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Novartis | BRETHAIRE | terbutaline sulfate | AEROSOL, METERED;INHALATION | 018762-001 | Aug 17, 1984 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Ani Pharms | BRETHINE | terbutaline sulfate | TABLET;ORAL | 017849-002 | Approved Prior to Jan 1, 1982 | AB | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Twi Pharms | TERBUTALINE SULFATE | terbutaline sulfate | TABLET;ORAL | 211832-002 | Jun 19, 2020 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Sanofi Aventis Us | BRICANYL | terbutaline sulfate | TABLET;ORAL | 017618-002 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Impax Labs | TERBUTALINE SULFATE | terbutaline sulfate | TABLET;ORAL | 075877-001 | Jun 26, 2001 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for terbutaline sulfate
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Ani Pharms | BRETHINE | terbutaline sulfate | TABLET;ORAL | 017849-001 | Approved Prior to Jan 1, 1982 | ⤷ Try a Trial | ⤷ Try a Trial |
| Sanofi Aventis Us | BRICANYL | terbutaline sulfate | INJECTABLE;INJECTION | 017466-001 | Approved Prior to Jan 1, 1982 | ⤷ Try a Trial | ⤷ Try a Trial |
| Sanofi Aventis Us | BRICANYL | terbutaline sulfate | AEROSOL, METERED;INHALATION | 018000-001 | Mar 19, 1985 | ⤷ Try a Trial | ⤷ Try a Trial |
| Sanofi Aventis Us | BRICANYL | terbutaline sulfate | TABLET;ORAL | 017618-002 | Approved Prior to Jan 1, 1982 | ⤷ Try a Trial | ⤷ Try a Trial |
| Sanofi Aventis Us | BRICANYL | terbutaline sulfate | AEROSOL, METERED;INHALATION | 018000-001 | Mar 19, 1985 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |