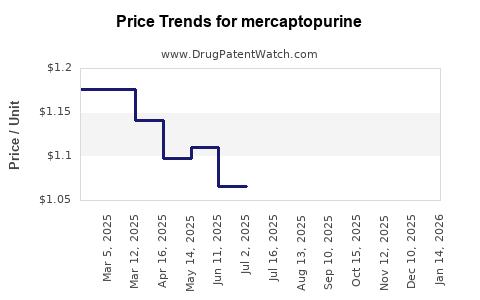
Last updated: July 27, 2025
Introduction
Mercaptopurine, marketed commercially under brand names such as Purinethol, is an established chemotherapeutic agent primarily used in the treatment of acute lymphoblastic leukemia (ALL) and certain other hematologic malignancies. A purine analog, it functions by inhibiting nucleotide synthesis, interfering with DNA replication in rapidly dividing cells. As the landscape of oncology treatment evolves, understanding the current market dynamics and projected financial trajectory of mercaptopurine is crucial for stakeholders, including pharmaceutical companies, investors, and healthcare providers.
Market Overview
The global oncology drugs market is witnessing unprecedented growth, driven by an increasing incidence of cancers, advances in targeted therapies, and rising healthcare expenditure. Within this landscape, generic versions of established chemotherapeutics like mercaptopurine have seen a significant uptick in adoption, especially in emerging markets. According to IQVIA data, the hematologic malignancies segment dominates the oncology therapeutics market, with mercaptopurine being a staple in chemotherapy regimens for pediatric and adult ALL cases.
Mercaptopurine’s role remains pivotal, especially as part of combination therapies. Nonetheless, the market shares of newer, targeted agents—such as tyrosine kinase inhibitors and monoclonal antibodies—pose competitive pressures. Yet, due to its long-standing efficacy, affordable cost, and inclusion in many treatment protocols, mercaptopurine maintains steady demand.
Market Drivers
Several factors underpin the current market dynamics:
-
Prevalence of Hematologic Cancers: According to the Global Cancer Statistics 2020,[1] leukemia accounts for approximately 3.2% of all new cancer cases globally, with pediatric ALL being a common subtype. Rising incidence rates sustain demand for chemotherapeutics like mercaptopurine.
-
Cost-Effectiveness and Accessibility: As a generic drug, mercaptopurine remains affordable, particularly in developing economies, augmenting its widespread use.
-
Established Efficacy and Safety Profile: Decades of clinical use bolster physician confidence, ensuring its continued role in treatment protocols.
-
Regulatory Environment: Favorable regulations for generic manufacturing in key markets facilitate steady supply.
Market Challenges
Despite its entrenched position, mercaptopurine faces several challenges:
-
Emergence of Targeted Therapies: Agents like blinatumomab and inotuzumab ozogamicin show promising efficacy, potentially displacing traditional chemotherapies in certain indications.[2]
-
Toxicity Profiles: Adverse effects such as myelosuppression necessitate careful dosing and monitoring, which may limit use in some patient populations.
-
Patient Compliance and Resistance: Long-term therapy may lead to resistance or intolerance, necessitating alternative options.
Financial Trajectory
Historical Perspective
The revenue generated from mercaptopurine has been relatively stable over the past decade, owing mainly to its low manufacturing costs and consistent demand. The global market for mercaptopurine and similar chemotherapeutics was valued at approximately USD 0.5 billion in 2020, with projections indicating modest growth at a compounded annual growth rate (CAGR) of around 3–4% over the next five years.[3]
Projected Growth
Factors influencing the financial outlook include:
-
Generic Market Expansion: The generics segment is expected to continue its growth trajectory, driven by patent expirations and increased manufacturing capacity. This sustains low pricing and high-volume sales, especially in resource-limited settings.
-
Geographical Expansion: Asia-Pacific and Latin America’s increasing healthcare infrastructure and cancer screening programs will propel demand. The Asia-Pacific region alone is projected to account for over 35% of the global oncology drug market by 2025.[4]
-
New Formulations and Combination Therapies: Development of improved formulations (e.g., suppositories, enhanced bioavailability forms) and combination therapies may open incremental revenue streams.
Despite these positive trends, competition from newer agents and stringent regulatory scrutiny could suppress profit margins, especially for branded versions.
Impact of Biosimilars and Generics
The entry of biosimilars and low-cost generics further influences the market by reducing prices and expanding access. A notable example is the proliferation of generic mercaptopurine formulations by multiple manufacturers globally, which precludes significant pricing power for original developers.
Regulatory and Patent Landscape
Most patents related to mercaptopurine have expired, leading to widespread generic manufacturing. Regulatory agencies have streamlined approval processes for generics and biosimilars, fostering increased market penetration.
However, ongoing patent litigations and quality assurance standards could impact supply chains and market stability. Continuous vigilance by industry participants is required to adapt to regulatory shifts and optimize revenue streams.
Competitive Landscape
The battle for market share predominantly involves generic manufacturers rather than branded pharmaceutical companies, given the drug's age. Top generic producers include Teva Pharmaceuticals, Mylan, and Sun Pharmaceutical Industries. Their strategies focus on manufacturing cost efficiencies, regulatory compliance, and expanding access in emerging markets.
Novel therapies aiming to replace or complement mercaptopurine face challenges in demonstrating superior efficacy, safety, and cost benefits. Nevertheless, personalized medicine initiatives could eventually reshape treatment protocols, impacting demand trajectories.
Future Outlook
In the foreseeable future, mercaptopurine’s financial performance hinges on several key factors:
-
Stable demand in existing treatments ensures a baseline revenue stream.
-
Market expansion into emerging economies offers growth potential, especially where healthcare infrastructure improves.
-
Potential shifts towards targeted agents could marginalize traditional chemotherapy, although cost advantages for mercaptopurine may mitigate this impact in lower-income regions.
-
Ongoing research might identify new indications, expanding its therapeutic scope or leading to combination therapies.
Overall, the financial trajectory points to modest but steady growth, sustained by generics and increasing global cancer burden.
Key Takeaways
-
Stable, modest growth expected, driven by generic manufacturing, expanding access in emerging markets, and rising cancer incidence.
-
Market dynamics complicated by competition from targeted therapies, but cost advantages and entrenched clinical use maintain demand.
-
Regulatory dynamics favor generics, with patent expirations facilitating entry and price competition.
-
Investments in new formulations and combination therapies could generate incremental revenues.
-
Global health initiatives and increasing screening programs will bolster demand, especially in low- and middle-income countries.
Conclusion
Mercaptopurine remains a cornerstone chemotherapeutic agent within hematology oncology. Its market continues to evolve against a backdrop of technological advancements, regulatory changes, and shifting institutional preferences. Stakeholders must balance the drug’s established efficacy and affordability with emerging competition and innovation to optimize their strategic positioning and capitalize on its ongoing financial potential.
FAQs
-
What are the primary indications for mercaptopurine?
Mercaptopurine is primarily indicated for acute lymphoblastic leukemia (ALL), both as a part of maintenance therapy and in combination regimens. It is also used for certain other hematological malignancies.
-
How does the patent status of mercaptopurine influence its market?
With most patents expired, the market is predominantly occupied by generic manufacturers, leading to increased competition, lower prices, and greater accessibility, especially in developing countries.
-
What are the main competitors to mercaptopurine in current oncology treatment?
While traditional chemotherapeutic agents remain in use, emerging targeted therapies and immunotherapies like blinatumomab and CAR-T cell therapies are becoming alternatives, particularly in relapsed or refractory cases.
-
What factors could impact the future profitability of mercaptopurine?
Key factors include the development of new targeted agents, regulatory changes, shifts in clinical practice, patent laws, and market expansion in underserved regions.
-
Are there ongoing research efforts enhancing mercaptopurine’s utility?
Yes, research is ongoing into combination therapies, novel formulations, and potential new indications, which could sustain or enhance its market relevance.
References
[1] Globocan 2020, International Agency for Research on Cancer.
[2] Jabbour et al., 2019, "Emerging therapies in acute lymphoblastic leukemia," Leukemia.
[3] Market Research Future, 2022, "Pharmaceuticals Market - Forecast to 2027."
[4] Grand View Research, 2021, "Oncology Drugs Market Size, Share & Trends."