Icosapent ethyl - Generic Drug Details
✉ Email this page to a colleague
What are the generic sources for icosapent ethyl and what is the scope of freedom to operate?
Icosapent ethyl
is the generic ingredient in two branded drugs marketed by Apotex, Ascent Pharms Inc, Dr Reddys, Hikma, Humanwell Puracap, Strides Pharma, Teva Pharms Usa, Zydus Lifesciences, and Amarin Pharms, and is included in nine NDAs. There are sixty-three patents protecting this compound and three Paragraph IV challenges. Additional information is available in the individual branded drug profile pages.Icosapent ethyl has three hundred and sixty-nine patent family members in forty-six countries.
There are six drug master file entries for icosapent ethyl. Fifteen suppliers are listed for this compound.
Summary for icosapent ethyl
| International Patents: | 369 |
| US Patents: | 63 |
| Tradenames: | 2 |
| Applicants: | 9 |
| NDAs: | 9 |
| Drug Master File Entries: | 6 |
| Finished Product Suppliers / Packagers: | 15 |
| Raw Ingredient (Bulk) Api Vendors: | 60 |
| Clinical Trials: | 17 |
| Patent Applications: | 2,164 |
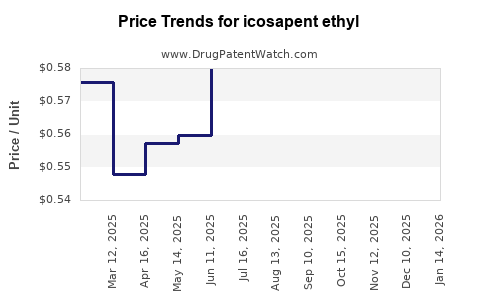
| Drug Prices: | Drug price trends for icosapent ethyl |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for icosapent ethyl |
| What excipients (inactive ingredients) are in icosapent ethyl? | icosapent ethyl excipients list |
| DailyMed Link: | icosapent ethyl at DailyMed |
Recent Clinical Trials for icosapent ethyl
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| National Cancer Institute (NCI) | Phase 1/Phase 2 |
| University of Western Ontario, Canada | Phase 4 |
| HLS Therapeutics, Inc | Phase 4 |
Paragraph IV (Patent) Challenges for ICOSAPENT ETHYL
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| VASCEPA | Capsules | icosapent ethyl | 500 mg | 202057 | 1 | 2017-08-29 |
| VASCEPA | Capsules | icosapent ethyl | 1 g | 202057 | 4 | 2016-07-26 |
US Patents and Regulatory Information for icosapent ethyl
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amarin Pharms | VASCEPA | icosapent ethyl | CAPSULE;ORAL | 202057-001 | Jul 26, 2012 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Amarin Pharms | VASCEPA | icosapent ethyl | CAPSULE;ORAL | 202057-002 | Feb 16, 2017 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Amarin Pharms | VASCEPA | icosapent ethyl | CAPSULE;ORAL | 202057-001 | Jul 26, 2012 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Amarin Pharms | VASCEPA | icosapent ethyl | CAPSULE;ORAL | 202057-002 | Feb 16, 2017 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Amarin Pharms | VASCEPA | icosapent ethyl | CAPSULE;ORAL | 202057-001 | Jul 26, 2012 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Amarin Pharms | VASCEPA | icosapent ethyl | CAPSULE;ORAL | 202057-001 | Jul 26, 2012 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Amarin Pharms | VASCEPA | icosapent ethyl | CAPSULE;ORAL | 202057-001 | Jul 26, 2012 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for icosapent ethyl
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Amarin Pharms | VASCEPA | icosapent ethyl | CAPSULE;ORAL | 202057-002 | Feb 16, 2017 | ⤷ Sign Up | ⤷ Sign Up |
| Amarin Pharms | VASCEPA | icosapent ethyl | CAPSULE;ORAL | 202057-001 | Jul 26, 2012 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for icosapent ethyl
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Amarin Pharmaceuticals Ireland Limited | Vazkepa | icosapent ethyl | EMEA/H/C/005398 Indicated to reduce cardiovascular risk as an adjunct to statin therapy. |
Authorised | no | no | no | 2021-03-26 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for icosapent ethyl
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| European Patent Office | 3278665 | COMPOSITION PHARMACEUTIQUE STABLE ET PROCÉDÉS D'UTILISATION ASSOCIÉS (STABLE PHARMACEUTICAL COMPOSITION AND METHODS OF USING SAME) | ⤷ Sign Up |
| World Intellectual Property Organization (WIPO) | 2010147994 | ⤷ Sign Up | |
| Portugal | 3815684 | ⤷ Sign Up | |
| Cyprus | 1122628 | ⤷ Sign Up | |
| Spain | 2426132 | ⤷ Sign Up | |
| Poland | 3318255 | ⤷ Sign Up | |
| Eurasian Patent Organization | 202190859 | СПОСОБЫ СНИЖЕНИЯ РИСКА СЕРДЕЧНО-СОСУДИСТЫХ СОБЫТИЙ У СУБЪЕКТА | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for icosapent ethyl
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2443246 | SPC/GB21/056 | United Kingdom | ⤷ Sign Up | PRODUCT NAME: ICOSAPENT ETHYL; REGISTERED: UK EU/1/20/1524(FOR NI) 20210329; UK FURTHER MA ON IPSUM 20210329 |
| 2443246 | CA 2021 00036 | Denmark | ⤷ Sign Up | PRODUCT NAME: ICOSAPENT ETHYL; REG. NO/DATE: EU/1/20/1524 20210329 |
| 2443246 | 2021C/538 | Belgium | ⤷ Sign Up | PRODUCT NAME: VAZKEPA - ICOSAPENT ETHYL; AUTHORISATION NUMBER AND DATE: EU/1/20/1524 20210329 |
| 2443246 | C202130052 | Spain | ⤷ Sign Up | PRODUCT NAME: ICOSAPENTO DE ETILO; NATIONAL AUTHORISATION NUMBER: EU/1/20/1524; DATE OF AUTHORISATION: 20210326; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/20/1524; DATE OF FIRST AUTHORISATION IN EEA: 20210326 |
| 2443246 | 21C1046 | France | ⤷ Sign Up | PRODUCT NAME: ICOSAPENT ETHYL; NAT. REGISTRATION NO/DATE: EU/1/20/1524 20210329; FIRST REGISTRATION: - EU/1/20/1524 20210329 |
| 2022495 | 21C1045 | France | ⤷ Sign Up | PRODUCT NAME: ICOSAPENT ETHYL; REGISTRATION NO/DATE: EU/1/20/1524 20210329 |
| 2443246 | 122021000056 | Germany | ⤷ Sign Up | PRODUCT NAME: VAZKEPA (ICOSAPENT ETHYL); REGISTRATION NO/DATE: EU/1/20/1524 20210326 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |