Capsaicin - Generic Drug Details
✉ Email this page to a colleague
What are the generic drug sources for capsaicin and what is the scope of freedom to operate?
Capsaicin
is the generic ingredient in one branded drug marketed by Averitas and is included in one NDA. There are three patents protecting this compound. Additional information is available in the individual branded drug profile pages.Capsaicin has sixty-seven patent family members in twenty-seven countries.
There are six drug master file entries for capsaicin. One supplier is listed for this compound.
Summary for capsaicin
| International Patents: | 67 |
| US Patents: | 3 |
| Tradenames: | 1 |
| Applicants: | 1 |
| NDAs: | 1 |
| Drug Master File Entries: | 6 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 150 |
| Clinical Trials: | 196 |
| Patent Applications: | 6,470 |
| Formulation / Manufacturing: | see details |
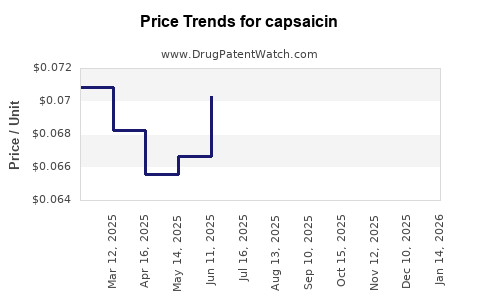
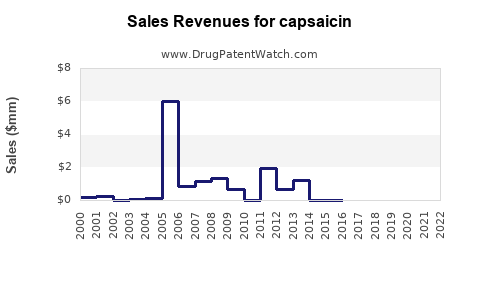
| Drug Prices: | Drug price trends for capsaicin |
| What excipients (inactive ingredients) are in capsaicin? | capsaicin excipients list |
| DailyMed Link: | capsaicin at DailyMed |
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for capsaicin
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for capsaicin
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| University Hospital, Brest | Phase 2 |
| Nantes University Hospital | Phase 3 |
| Federal University of Minas Gerais | Phase 4 |
Medical Subject Heading (MeSH) Categories for capsaicin
Anatomical Therapeutic Chemical (ATC) Classes for capsaicin
US Patents and Regulatory Information for capsaicin
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Averitas | QUTENZA | capsaicin | PATCH;TOPICAL | 022395-001 | Nov 16, 2009 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Averitas | QUTENZA | capsaicin | PATCH;TOPICAL | 022395-001 | Nov 16, 2009 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Averitas | QUTENZA | capsaicin | PATCH;TOPICAL | 022395-001 | Nov 16, 2009 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for capsaicin
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Averitas | QUTENZA | capsaicin | PATCH;TOPICAL | 022395-001 | Nov 16, 2009 | ⤷ Sign Up | ⤷ Sign Up |
| Averitas | QUTENZA | capsaicin | PATCH;TOPICAL | 022395-001 | Nov 16, 2009 | ⤷ Sign Up | ⤷ Sign Up |
| Averitas | QUTENZA | capsaicin | PATCH;TOPICAL | 022395-001 | Nov 16, 2009 | ⤷ Sign Up | ⤷ Sign Up |
| Averitas | QUTENZA | capsaicin | PATCH;TOPICAL | 022395-001 | Nov 16, 2009 | ⤷ Sign Up | ⤷ Sign Up |
| Averitas | QUTENZA | capsaicin | PATCH;TOPICAL | 022395-001 | Nov 16, 2009 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for capsaicin
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Grunenthal GmbH | Qutenza | capsaicin | EMEA/H/C/000909 Qutenza is indicated for the treatment of peripheral neuropathic pain in adults either alone or in combination with other medicinal products for pain. |
Authorised | no | no | no | 2009-05-15 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for capsaicin
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Spain | 2288222 | ⤷ Sign Up | |
| China | 102755305 | Transdermal therapeutic system comprising an adhesive layer method for siliconizing the back layer of the system and use of said back layer | ⤷ Sign Up |
| Russian Federation | 2005134984 | ТЕРАПЕВТИЧЕСКИЙ ПЛАСТЫРЬ С ПОЛИСИЛОКСАНОВОЙ МАТРИЦЕЙ, СОДЕРЖАЩЕЙ КАПСАИЦИН | ⤷ Sign Up |
| South Africa | 200506462 | Therapeutic patch with polysiloxane matrix comprising capsaicin | ⤷ Sign Up |
| China | 1784222 | Therapeutic patch with polysiloxane matrix comprising capsaicin | ⤷ Sign Up |
| Cyprus | 1113997 | ⤷ Sign Up | |
| Russian Federation | 2007113674 | ТРАНСДЕРМАЛЬНАЯ ТЕРАПЕВТИЧЕСКАЯ СИСТЕМА С АДГЕЗИОННЫМ СЛОЕМ, СПОСОБ СИЛИКОНИЗАЦИИ СЛОЯ ОСНОВЫ СИСТЕМЫ И ПРИМЕНЕНИЕ СЛОЯ ОСНОВЫ | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |