Armodafinil - Generic Drug Details
✉ Email this page to a colleague
What are the generic sources for armodafinil and what is the scope of freedom to operate?
Armodafinil
is the generic ingredient in two branded drugs marketed by Aurobindo Pharma Ltd, Corepharma, Lupin Ltd, Mylan Pharms Inc, Natco Pharma Ltd, Watson Labs Inc, and Cephalon, and is included in seven NDAs. There are two patents protecting this compound and two Paragraph IV challenges. Additional information is available in the individual branded drug profile pages.Armodafinil has one hundred and sixteen patent family members in thirty-one countries.
There are twelve drug master file entries for armodafinil. Twelve suppliers are listed for this compound. There are three tentative approvals for this compound.
Summary for armodafinil
| International Patents: | 116 |
| US Patents: | 2 |
| Tradenames: | 2 |
| Applicants: | 7 |
| NDAs: | 7 |
| Drug Master File Entries: | 12 |
| Finished Product Suppliers / Packagers: | 12 |
| Raw Ingredient (Bulk) Api Vendors: | 36 |
| Clinical Trials: | 59 |
| Patent Applications: | 5,770 |
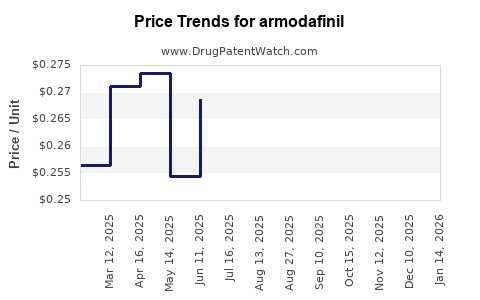
| Drug Prices: | Drug price trends for armodafinil |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for armodafinil |
| What excipients (inactive ingredients) are in armodafinil? | armodafinil excipients list |
| DailyMed Link: | armodafinil at DailyMed |
Recent Clinical Trials for armodafinil
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Charles Andrew Czeisler, MD, PhD | Phase 4 |
| Jazz Pharmaceuticals | Phase 4 |
| University of Florida | Phase 1 |
Generic filers with tentative approvals for ARMODAFINIL
The 'tentative' approval signifies that the product meets all FDA standards for marketing, and, but for the patents / regulatory protections, it would approved.
Anatomical Therapeutic Chemical (ATC) Classes for armodafinil
Paragraph IV (Patent) Challenges for ARMODAFINIL
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| NUVIGIL | Tablets | armodafinil | 100 mg | 021875 | 1 | 2009-09-08 |
| NUVIGIL | Tablets | armodafinil | 200 mg | 021875 | 1 | 2009-09-03 |
| NUVIGIL | Tablets | armodafinil | 50 mg, 150 mg and 250 mg | 021875 | 1 | 2009-07-24 |
US Patents and Regulatory Information for armodafinil
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Corepharma | ARMODAFINIL | armodafinil | TABLET;ORAL | 201514-002 | Mar 25, 2019 | DISCN | No | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Watson Labs Inc | ARMODAFINIL | armodafinil | TABLET;ORAL | 200156-004 | Aug 29, 2012 | DISCN | No | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Aurobindo Pharma Ltd | ARMODAFINIL | armodafinil | TABLET;ORAL | 206069-002 | Mar 6, 2018 | AB | RX | No | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for armodafinil
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Cephalon | NUVIGIL | armodafinil | TABLET;ORAL | 021875-003 | Jun 15, 2007 | ⤷ Sign Up | ⤷ Sign Up |
| Cephalon | NUVIGIL | armodafinil | TABLET;ORAL | 021875-003 | Jun 15, 2007 | ⤷ Sign Up | ⤷ Sign Up |
| Cephalon | NUVIGIL | armodafinil | TABLET;ORAL | 021875-004 | Jun 15, 2007 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for armodafinil
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| World Intellectual Property Organization (WIPO) | 02096401 | ⤷ Sign Up | |
| Japan | 4719471 | ⤷ Sign Up | |
| Eurasian Patent Organization | 008506 | НОВЫЕ ФАРМАЦЕВТИЧЕСКИЕ СОСТАВЫ МОДАФИНИЛА (NOVEL PHARMACEUTICAL FORMULATIONS OF MODAFINIL) | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |