SANDOZ Company Profile
✉ Email this page to a colleague
What is the competitive landscape for SANDOZ, and when can generic versions of SANDOZ drugs launch?
SANDOZ has four hundred and eighty-nine approved drugs.
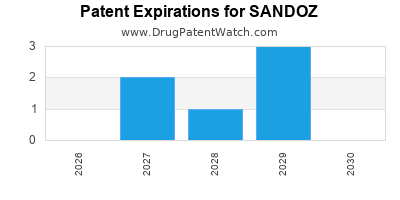
There are nine US patents protecting SANDOZ drugs. There are thirty-three tentative approvals on SANDOZ drugs.
There are one hundred and three patent family members on SANDOZ drugs in thirty-four countries and five hundred and four supplementary protection certificates in seventeen countries.
Summary for SANDOZ
| International Patents: | 103 |
| US Patents: | 9 |
| Tradenames: | 322 |
| Ingredients: | 300 |
| NDAs: | 489 |
| Patent Litigation for SANDOZ: | See patent lawsuits for SANDOZ |
| PTAB Cases with SANDOZ as petitioner: | See PTAB cases with SANDOZ as petitioner |
Drugs and US Patents for SANDOZ
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sandoz | DICLOXACILLIN SODIUM | dicloxacillin sodium | CAPSULE;ORAL | 061454-003 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Sandoz | PROPOXYPHENE HYDROCHLORIDE | propoxyphene hydrochloride | CAPSULE;ORAL | 083688-001 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Sandoz | DISOBROM | dexbrompheniramine maleate; pseudoephedrine sulfate | TABLET, EXTENDED RELEASE;ORAL | 070770-001 | Sep 30, 1991 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Sandoz | CARISOPRODOL, ASPIRIN AND CODEINE PHOSPHATE | aspirin; carisoprodol; codeine phosphate | TABLET;ORAL | 040118-001 | Apr 16, 1996 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for SANDOZ
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Sandoz | FOCALIN XR | dexmethylphenidate hydrochloride | CAPSULE, EXTENDED RELEASE;ORAL | 021802-002 | May 26, 2005 | 6,528,530 | ⤷ Try a Trial |
| Sandoz | VIVELLE-DOT | estradiol | SYSTEM;TRANSDERMAL | 020538-009 | May 3, 2002 | 6,024,976 | ⤷ Try a Trial |
| Sandoz | FOCALIN XR | dexmethylphenidate hydrochloride | CAPSULE, EXTENDED RELEASE;ORAL | 021802-001 | May 26, 2005 | 6,528,530 | ⤷ Try a Trial |
| Sandoz | HYCAMTIN | topotecan hydrochloride | CAPSULE;ORAL | 020981-001 | Oct 11, 2007 | 5,004,758*PED | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for SANDOZ drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Capsules | 5 mg/40 mg and 10 mg/40 mg | ➤ Subscribe | 2006-11-17 |
| ➤ Subscribe | Capsules | 1.5 mg, 3 mg, 4.5 mg and 6 mg | ➤ Subscribe | 2004-04-21 |
| ➤ Subscribe | Transdermal System Extended-release | 13.3 mg/24 hr | ➤ Subscribe | 2013-01-22 |
| ➤ Subscribe | Injection | 1 mg/mL, 50 mL vials | ➤ Subscribe | 2011-12-16 |
| ➤ Subscribe | Extended-release Capsules | 15 mg | ➤ Subscribe | 2007-05-14 |
| ➤ Subscribe | For Injection | 250 mg/vial | ➤ Subscribe | 2009-09-01 |
| ➤ Subscribe | Extended-release Capsule | 30 mg | ➤ Subscribe | 2010-12-15 |
| ➤ Subscribe | Ophthalmic Solution | 0.00% | ➤ Subscribe | 2009-02-19 |
| ➤ Subscribe | Extended-release capsules | 25 mg | ➤ Subscribe | 2011-09-30 |
| ➤ Subscribe | Extended-release Tablets | 80 mg | ➤ Subscribe | 2007-03-15 |
| ➤ Subscribe | Extended-release Capsules | 10 mg | ➤ Subscribe | 2007-05-21 |
| ➤ Subscribe | Tablets | 5 mg and 10 mg | ➤ Subscribe | 2004-05-27 |
| ➤ Subscribe | Capsules | 2.5 mg/10 mg, 5 mg/10 mg, 5 mg/20 mg and 10 mg/20 mg | ➤ Subscribe | 2004-06-09 |
| ➤ Subscribe | Ophthalmic Emulsion | 0.05% | ➤ Subscribe | 2014-05-01 |
| ➤ Subscribe | Oral Solution | 2 mg/mL | ➤ Subscribe | 2004-11-05 |
| ➤ Subscribe | Injection | 100 mg/mL, 2.5 mL vials | ➤ Subscribe | 2007-09-24 |
| ➤ Subscribe | Delayed-release Tablets | 20 mg | ➤ Subscribe | 2015-06-03 |
| ➤ Subscribe | Oral Solution | 4 mg/5 mL | ➤ Subscribe | 2004-12-20 |
| ➤ Subscribe | Extended-release Capsules | 5mg, 10mg and 20 mg | ➤ Subscribe | 2007-03-30 |
| ➤ Subscribe | Injection | 0.05 mg/mL, 100 mL vial | ➤ Subscribe | 2008-08-29 |
| ➤ Subscribe | Extended-release Capsule | 40 mg | ➤ Subscribe | 2010-12-20 |
| ➤ Subscribe | Otic Suspension | 0.3%/0.1% | ➤ Subscribe | 2012-07-31 |
| ➤ Subscribe | Extended-release capsules | 35 mg | ➤ Subscribe | 2011-09-29 |
| ➤ Subscribe | Tablets | 2.5 mg | ➤ Subscribe | 2004-07-27 |
| ➤ Subscribe | Extended-release Capsules | 20 mg, 30 mg and 40 mg | ➤ Subscribe | 2006-08-21 |
International Patents for SANDOZ Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| World Intellectual Property Organization (WIPO) | 2005046608 | ⤷ Try a Trial |
| South Korea | 101421519 | ⤷ Try a Trial |
| Australia | 2007299727 | ⤷ Try a Trial |
| European Patent Office | 2066300 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for SANDOZ Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0287150 | 96C0038 | Belgium | ⤷ Try a Trial | PRODUCT NAME: ROCURONIUMBROMIDE; NAT. REGISTRATION NO/DATE: 63 IS 9 F 12 19960709; FIRST REGISTRATION: NL RVG 16946 19940406 |
| 0734381 | SPC/GB04/011 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: APREPITANT, OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT; REGISTERED: UK EU/1/03/262/001 20031113; UK EU/1/03/262/002 20031113; UK EU/1/03/262/003 20031113; UK EU/1/03/262/004 20031113; UK EU/1/03/262/005 20031113; UK EU/1/03/262/006 20031113 |
| 2217577 | LUC00103 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: DUZALLO - ALLOPURINOL/LESINURAD OU UN/DES SEL(S) PHARMACEUTIQUEMENT ACCEPTABLE(S) DE CELUI-CI; AUTHORISATION NUMBER AND DATE: EU/1/18/1300 20180827 |
| 1781277 | PA2024501 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: IBUPROFENO IR PARACETAMOLIO DERINYS; REGISTRATION NO/DATE: LT/1/23/5212/001-002 20230726 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.