PF PRISM CV Company Profile
✉ Email this page to a colleague
What is the competitive landscape for PF PRISM CV, and when can generic versions of PF PRISM CV drugs launch?
PF PRISM CV has sixteen approved drugs.
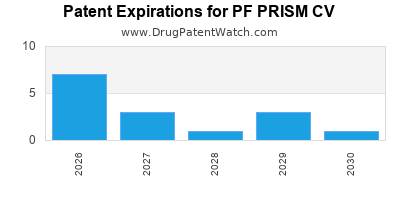
There are twenty-nine US patents protecting PF PRISM CV drugs.
There are six hundred and twelve patent family members on PF PRISM CV drugs in sixty-six countries and seventy-two supplementary protection certificates in eighteen countries.
Summary for PF PRISM CV
| International Patents: | 612 |
| US Patents: | 29 |
| Tradenames: | 11 |
| Ingredients: | 11 |
| NDAs: | 16 |
Drugs and US Patents for PF PRISM CV
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pf Prism Cv | XALKORI | crizotinib | CAPSULE;ORAL | 202570-002 | Aug 26, 2011 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Pf Prism Cv | XALKORI | crizotinib | CAPSULE;ORAL | 202570-001 | Aug 26, 2011 | RX | Yes | No | 8,217,057 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Pf Prism Cv | VFEND | voriconazole | INJECTABLE;INTRAVENOUS | 021267-001 | May 24, 2002 | AP | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Pf Prism Cv | BOSULIF | bosutinib monohydrate | CAPSULE;ORAL | 217729-001 | Sep 26, 2023 | RX | Yes | No | 11,103,497*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for PF PRISM CV
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Pf Prism Cv | RAPAMUNE | sirolimus | TABLET;ORAL | 021110-002 | Aug 22, 2002 | 5,212,155*PED | ⤷ Try a Trial |
| Pf Prism Cv | RAPAMUNE | sirolimus | SOLUTION;ORAL | 021083-001 | Sep 15, 1999 | 5,100,899*PED | ⤷ Try a Trial |
| Pf Prism Cv | TORISEL | temsirolimus | SOLUTION;INTRAVENOUS | 022088-001 | May 30, 2007 | 8,455,539*PED | ⤷ Try a Trial |
| Pf Prism Cv | BOSULIF | bosutinib monohydrate | TABLET;ORAL | 203341-003 | Oct 27, 2017 | 6,002,008 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for PF PRISM CV drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Tablets | 400 mg | ➤ Subscribe | 2018-10-15 |
| ➤ Subscribe | Tablets | 50 mg and 200 mg | ➤ Subscribe | 2008-04-14 |
| ➤ Subscribe | For Injection | 50 mg per vial | ➤ Subscribe | 2009-06-15 |
| ➤ Subscribe | Tablets | 1 mg and 2 mg | ➤ Subscribe | 2009-12-17 |
| ➤ Subscribe | Extended-release Tablets | 50 mg and 100 mg | ➤ Subscribe | 2012-02-29 |
| ➤ Subscribe | Tablets | 0.5 mg and 1 mg | ➤ Subscribe | 2010-05-10 |
| ➤ Subscribe | Tablets | 5 mg | ➤ Subscribe | 2016-11-07 |
| ➤ Subscribe | Tablets | 100mg and 500mg | ➤ Subscribe | 2016-09-06 |
| ➤ Subscribe | For Injection | 200 mg/vial | ➤ Subscribe | 2008-09-12 |
| ➤ Subscribe | Oral Suspension | 40 mg/mL | ➤ Subscribe | 2010-10-08 |
| ➤ Subscribe | Injection | 25 mg/mL, 1.8 mL vial | ➤ Subscribe | 2011-05-25 |
| ➤ Subscribe | Tablets | 0.5 mg | ➤ Subscribe | 2010-08-25 |
| ➤ Subscribe | Extended-release Tablets | 25 mg | ➤ Subscribe | 2015-05-08 |
| ➤ Subscribe | Tablets | 1 mg and 5 mg | ➤ Subscribe | 2018-02-23 |
International Patents for PF PRISM CV Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Argentina | 065931 | ⤷ Try a Trial |
| Guatemala | 200600282 | ⤷ Try a Trial |
| Denmark | 2462934 | ⤷ Try a Trial |
| Israel | 201320 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for PF PRISM CV Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1786785 | 12/2013 | Austria | ⤷ Try a Trial | PRODUCT NAME: CRIZOTINIB, BEVORZUGT IN FORM EINES PHARMAZEUTISCH AKZEPTABLEN SALZES, HYDRATS ODER SOLVATS; REGISTRATION NO/DATE: EU/1/12/793/001 - EU/1/12/793/004 20121023 |
| 1666481 | CA 2017 00035 | Denmark | ⤷ Try a Trial | PRODUCT NAME: TOFACITINIB, EVENTUELT I FORM AF ET FARMACEUTISK ACCEPTABELT SALT, HERUNDER CITRAT-SALTET; REG. NO/DATE: EU/1/17/1178/001-003 20170324 |
| 1666481 | 132017000095300 | Italy | ⤷ Try a Trial | PRODUCT NAME: TOFACITINIB, OPZIONALMENTE NELLA FORMA DI UN SALE FARMACEUTICAMENTE ACCETTABILE, COMPRENDENTE IL SALE CITRATO(XELJANZ); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/17/1178/001-004, 20170324 |
| 1786785 | C300587 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: CRIZOTINIB, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT, HYDRAAT OF SOLVAAT; REGISTRATION NO/DATE: EU/1/12/793/001-004 20121023 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.