Kaleo Inc Company Profile
✉ Email this page to a colleague
What is the competitive landscape for KALEO INC, and what generic alternatives to KALEO INC drugs are available?
KALEO INC has four approved drugs.
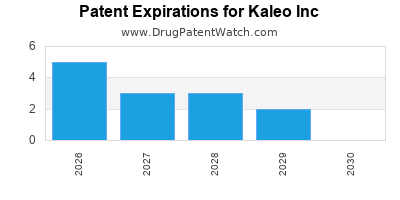
There are thirty-nine US patents protecting KALEO INC drugs.
There are one hundred and forty-four patent family members on KALEO INC drugs in fifteen countries and one supplementary protection certificate in one country.
Drugs and US Patents for Kaleo Inc
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kaleo Inc | AUVI-Q | epinephrine | SOLUTION;INTRAMUSCULAR, SUBCUTANEOUS | 201739-001 | Aug 10, 2012 | BX | RX | Yes | No | 9,737,669 | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Kaleo Inc | AUVI-Q | epinephrine | SOLUTION;INTRAMUSCULAR, SUBCUTANEOUS | 201739-002 | Aug 10, 2012 | BX | RX | Yes | Yes | 8,226,610 | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Kaleo Inc | AUVI-Q | epinephrine | SOLUTION;INTRAMUSCULAR, SUBCUTANEOUS | 201739-003 | Nov 17, 2017 | RX | Yes | No | 11,590,286 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Kaleo Inc | EVZIO (AUTOINJECTOR) | naloxone hydrochloride | SOLUTION;INTRAMUSCULAR, SUBCUTANEOUS | 209862-001 | Oct 19, 2016 | DISCN | Yes | No | 10,322,239 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Kaleo Inc | AUVI-Q | epinephrine | SOLUTION;INTRAMUSCULAR, SUBCUTANEOUS | 201739-003 | Nov 17, 2017 | RX | Yes | No | 8,425,462 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Kaleo Inc | EVZIO (AUTOINJECTOR) | naloxone hydrochloride | SOLUTION;INTRAMUSCULAR, SUBCUTANEOUS | 209862-001 | Oct 19, 2016 | DISCN | Yes | No | 8,425,462 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Kaleo Inc | NALOXONE HYDROCHLORIDE (AUTOINJECTOR) | naloxone hydrochloride | SOLUTION;INTRAMUSCULAR, SUBCUTANEOUS | 215457-001 | Feb 28, 2022 | DISCN | Yes | No | 10,322,239 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for Kaleo Inc Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| World Intellectual Property Organization (WIPO) | 2012118729 | ⤷ Try a Trial |
| Mexico | 2010012334 | ⤷ Try a Trial |
| Poland | 2058020 | ⤷ Try a Trial |
| Israel | 184552 | ⤷ Try a Trial |
| European Patent Office | 1814611 | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2008064092 | ⤷ Try a Trial |
| Japan | 7014797 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Kaleo Inc Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1685839 | 92292 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: COMBINAISON D OXYCODONE EN TANT QUE COMPOSANT A ET DE NALOXONE EN TANT QUE COMPOSANT B SOUS TOUTES LES FORMES PROTEGES PAR LE BREVET DE BASE |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.