Intercept Pharms Inc Company Profile
✉ Email this page to a colleague
What is the competitive landscape for INTERCEPT PHARMS INC, and what generic alternatives to INTERCEPT PHARMS INC drugs are available?
INTERCEPT PHARMS INC has one approved drug.
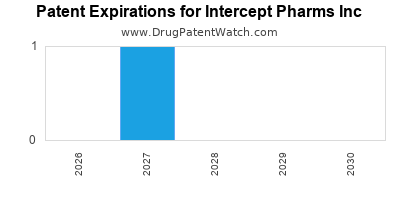
There are seven US patents protecting INTERCEPT PHARMS INC drugs.
There are one hundred and seventeen patent family members on INTERCEPT PHARMS INC drugs in thirty-seven countries and twelve supplementary protection certificates in eleven countries.
Summary for Intercept Pharms Inc
| International Patents: | 117 |
| US Patents: | 7 |
| Tradenames: | 1 |
| Ingredients: | 1 |
| NDAs: | 1 |
Drugs and US Patents for Intercept Pharms Inc
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intercept Pharms Inc | OCALIVA | obeticholic acid | TABLET;ORAL | 207999-001 | May 27, 2016 | RX | Yes | No | 10,758,549 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Intercept Pharms Inc | OCALIVA | obeticholic acid | TABLET;ORAL | 207999-002 | May 27, 2016 | RX | Yes | Yes | 10,052,337 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Intercept Pharms Inc | OCALIVA | obeticholic acid | TABLET;ORAL | 207999-002 | May 27, 2016 | RX | Yes | Yes | 9,238,673 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Intercept Pharms Inc | OCALIVA | obeticholic acid | TABLET;ORAL | 207999-001 | May 27, 2016 | RX | Yes | No | 10,052,337 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Intercept Pharms Inc | OCALIVA | obeticholic acid | TABLET;ORAL | 207999-001 | May 27, 2016 | RX | Yes | No | RE48286 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Intercept Pharms Inc | OCALIVA | obeticholic acid | TABLET;ORAL | 207999-002 | May 27, 2016 | RX | Yes | Yes | 10,751,349 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Intercept Pharms Inc | OCALIVA | obeticholic acid | TABLET;ORAL | 207999-002 | May 27, 2016 | RX | Yes | Yes | 10,047,117 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Intercept Pharms Inc
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Intercept Pharms Inc | OCALIVA | obeticholic acid | TABLET;ORAL | 207999-002 | May 27, 2016 | 8,377,916 | ⤷ Try a Trial |
| Intercept Pharms Inc | OCALIVA | obeticholic acid | TABLET;ORAL | 207999-002 | May 27, 2016 | 8,058,267 | ⤷ Try a Trial |
| Intercept Pharms Inc | OCALIVA | obeticholic acid | TABLET;ORAL | 207999-001 | May 27, 2016 | 8,377,916 | ⤷ Try a Trial |
| Intercept Pharms Inc | OCALIVA | obeticholic acid | TABLET;ORAL | 207999-001 | May 27, 2016 | 7,138,390 | ⤷ Try a Trial |
| Intercept Pharms Inc | OCALIVA | obeticholic acid | TABLET;ORAL | 207999-001 | May 27, 2016 | 8,058,267 | ⤷ Try a Trial |
| Intercept Pharms Inc | OCALIVA | obeticholic acid | TABLET;ORAL | 207999-002 | May 27, 2016 | 7,138,390 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for Intercept Pharms Inc Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| New Zealand | 703072 | ⤷ Try a Trial |
| Norway | 20034011 | ⤷ Try a Trial |
| Japan | 2018184443 | ⤷ Try a Trial |
| Portugal | 3336097 | ⤷ Try a Trial |
| Denmark | 3336097 | ⤷ Try a Trial |
| European Patent Office | 1392714 | ⤷ Try a Trial |
| Japan | 4021327 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Intercept Pharms Inc Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1392714 | 300877 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: OBETICHOLZUUR; REGISTRATION NO/DATE: EU/1/16/1139 20161215 |
| 1392714 | C01392714/01 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: ACIDUM OBETICHOLICUM; REGISTRATION NO/DATE: SWISSMEDIC-ZULASSUNG 66530 01.06.2018 |
| 1392714 | 1790020-0 | Sweden | ⤷ Try a Trial | PRODUCT NAME: OBETICHOLIC ACID; REG. NO/DATE: EU/1/16/1139 20161215 |
| 1392714 | 23/2017 | Austria | ⤷ Try a Trial | PRODUCT NAME: OBETICHOLSAEURE, DESSEN PHARMAZEUTISCH AKZEPTABLEN SALZE, SOLVATE ODER AMINOSAEURE-KONJUGATE; REGISTRATION NO/DATE: EU/1/16/1139 20161212 |
| 1392714 | 598 | Finland | ⤷ Try a Trial | |
| 1392714 | 17C0003 | France | ⤷ Try a Trial | PRODUCT NAME: ACIDE OBETICHOLIQUE; REGISTRATION NO/DATE: EU/1/16/1139 20161212 |
| 1392714 | 132017000061826 | Italy | ⤷ Try a Trial | PRODUCT NAME: ACIDO OBETICOLICO(OCALIVA); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/16/1139, 20161215 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.