PAR PHARM INC Company Profile
✉ Email this page to a colleague
What is the competitive landscape for PAR PHARM INC, and when can generic versions of PAR PHARM INC drugs launch?
PAR PHARM INC has thirty-eight approved drugs.
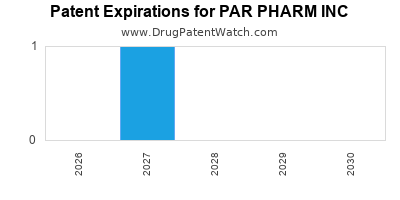
There is one US patent protecting PAR PHARM INC drugs. There are seven tentative approvals on PAR PHARM INC drugs.
There are nine patent family members on PAR PHARM INC drugs in eight countries and sixty-one supplementary protection certificates in fourteen countries.
Drugs and US Patents for PAR PHARM INC
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Par Pharm Inc | ALOSETRON HYDROCHLORIDE | alosetron hydrochloride | TABLET;ORAL | 206113-002 | Feb 23, 2018 | DISCN | No | No | ⤷ Sign Up | ⤷ Sign Up | |||||
| Par Pharm Inc | TIOPRONIN | tiopronin | TABLET;ORAL | 216198-001 | Jun 2, 2022 | DISCN | No | No | ⤷ Sign Up | ⤷ Sign Up | |||||
| Par Pharm Inc | TIZANIDINE HYDROCHLORIDE | tizanidine hydrochloride | TABLET;ORAL | 207170-001 | Jan 26, 2017 | DISCN | No | No | ⤷ Sign Up | ⤷ Sign Up | |||||
| Par Pharm Inc | MIDODRINE HYDROCHLORIDE | midodrine hydrochloride | TABLET;ORAL | 207169-001 | Oct 29, 2018 | AB | RX | No | No | ⤷ Sign Up | ⤷ Sign Up | ||||
| Par Pharm Inc | ZOLPIDEM TARTRATE | zolpidem tartrate | TABLET;SUBLINGUAL | 204229-001 | Sep 11, 2017 | AB | RX | No | No | ⤷ Sign Up | ⤷ Sign Up | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for PAR PHARM INC Drugs
Supplementary Protection Certificates for PAR PHARM INC Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1915993 | 300625 | Netherlands | ⤷ Sign Up | PRODUCT NAME: COMBINATIE BEVATTENDE ALISKIREN, OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN, EN AMLODIPINE, OF EEN FARMACEUATISCH AANVAARDBAAR ZOUT DAARVAN; REGISTRATION NO/DATE: EU/1/11/686/001-056 20110414 |
| 2435024 | 2190014-7 | Sweden | ⤷ Sign Up | PRODUCT NAME: A COMBINATION OF FORMOTEROL INCLUDING ANY PHARMACEUUTICALLY ACCEPTABLE SALTS, ESTERS, OR SOLVATES THEREOF, GLYCOPYRROLATE INCLUDING ANY PHARMACEUTICALLY ACCEPTABLE SALTS, ESTERS, OR SOLVATES THEREOF, AND BUDESONIDE INCLUDING ANY PHARMACEUTICALLY ACCEPTABLE SALT, ESTERS ORSOLVATES THEREOF; REG. NO/DATE: EU/1/20/1498 20201210 |
| 2435024 | SPC/GB21/029 | United Kingdom | ⤷ Sign Up | PRODUCT NAME: A COMBINATION OF FORMOTEROL, INCLUDING PHARMACEUTICALLY ACCEPTABLE SALTS, ESTERS AND SOLVATES THEREOF, GLYCOPYRROLATE, INCLUDING PHARMACEUTICALLY ACCEPTABLE SALTS, ESTERS AND SOLVATES THEREOF, AND BUDESONIDE INCLUDING PHARMACEUTICALLY ACCEPTABLE SALTS, ES; REGISTERED: UK EU/1/20/1498 (NI) 20201210; UK PLGB 17901/0352-001 20201210 |
| 2101777 | 2016C/032 | Belgium | ⤷ Sign Up | PRODUCT NAME: AMBRISENTAN EN COMBINAISON AVEC LE TADALAFIL; AUTHORISATION NUMBER AND DATE: EU/1/08/451 20151125 |
| 0785926 | SPC/GB08/047 | United Kingdom | ⤷ Sign Up | PRODUCT NAME: AMBRISENTAN; REGISTERED: UK EU/1/08/451/001 20080421; UK EU/1/08/451/002 20080421; UK EU/1/08/451/003 20080421; UK EU/1/08/451/004 20080421 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.