Which pharmaceutical companies have supplementary protection certificates in the most countries?
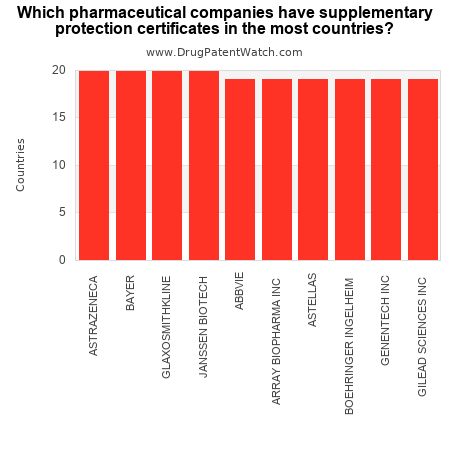
This chart shows the drug companies with the most supplementary protection certificates (SPCs).
SPCs are used in European Union and select others to encourage pharmaceutical innovation by compensating for the long time needed to obtain regulatory approval for drugs. SPCs come into force only after the corresponding general patent expires, and normally have a maximum lifetime of 5 years.
The total combined duration of market exclusivity of a general patent and SPC cannot normally exceed 15 years, but a six-month extention may be obtained by responding to a request for pediatric trials.
The pharma companies with SPCs in the most countries are:
