ABBVIE Company Profile
✉ Email this page to a colleague
What is the competitive landscape for ABBVIE, and what generic alternatives to ABBVIE drugs are available?
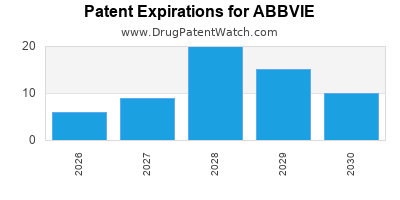
ABBVIE has one hundred and thirty-eight approved drugs.
There are two hundred and twenty-nine US patents protecting ABBVIE drugs.
There are two thousand seven hundred and twenty-two patent family members on ABBVIE drugs in sixty-six countries and three hundred and ninety-one supplementary protection certificates in nineteen countries.
Summary for ABBVIE
| International Patents: | 2722 |
| US Patents: | 229 |
| Tradenames: | 124 |
| Ingredients: | 99 |
| NDAs: | 138 |
| Drug Master File Entries: | 1 |
| Patent Litigation for ABBVIE: | See patent lawsuits for ABBVIE |
Drugs and US Patents for ABBVIE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbvie | UBRELVY | ubrogepant | TABLET;ORAL | 211765-001 | Dec 23, 2019 | RX | Yes | No | 9,499,545 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Abbvie | DUOPA | carbidopa; levodopa | SUSPENSION;ENTERAL | 203952-001 | Jan 9, 2015 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Abbvie | NALBUPHINE HYDROCHLORIDE | nalbuphine hydrochloride | INJECTABLE;INJECTION | 020200-001 | Mar 12, 1993 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for ABBVIE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Abbvie Endocrine Inc | LUPRON DEPOT-PED KIT | leuprolide acetate | POWDER;INTRAMUSCULAR | 020263-003 | Apr 16, 1993 | 4,728,721 | ⤷ Try a Trial |
| Abbvie | NORVIR | ritonavir | CAPSULE;ORAL | 020945-001 | Jun 29, 1999 | 7,141,593*PED | ⤷ Try a Trial |
| Abbvie | LINZESS | linaclotide | CAPSULE;ORAL | 202811-001 | Aug 30, 2012 | 8,110,553 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for ABBVIE drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Opthalmic Solution | 0.45% | ➤ Subscribe | 2011-08-23 |
| ➤ Subscribe | Capsules | 7 mg/10 mg | ➤ Subscribe | 2016-09-26 |
| ➤ Subscribe | Extended-release Tablets | 500 mg | ➤ Subscribe | 2005-02-08 |
| ➤ Subscribe | Ophthalmic Solution | 0.10% | ➤ Subscribe | 2006-12-20 |
| ➤ Subscribe | Oral Solution | 80 mg/20 mg per mL | ➤ Subscribe | 2014-06-19 |
| ➤ Subscribe | Extended-release Tablets | 2 mg/180 mg and 2 mg/240 mg | ➤ Subscribe | 2007-11-09 |
| ➤ Subscribe | Ophthalmic Solution | 0.2%/0.5% | ➤ Subscribe | 2008-11-21 |
| ➤ Subscribe | Tablets | 100 mg | ➤ Subscribe | 2010-12-21 |
| ➤ Subscribe | Extended-release Tablets | 4 mg/ 240 mg | ➤ Subscribe | 2007-07-24 |
| ➤ Subscribe | Ophthalmic Solution | 0.03% | ➤ Subscribe | 2008-12-22 |
| ➤ Subscribe | Tablets | 1 mg, 2 mg and 4 mg | ➤ Subscribe | 2004-10-04 |
| ➤ Subscribe | Injection | 0.002 mg per mL in 1 mL vial and 0.005 mg per mL in 1 mL and 2 mL vials | ➤ Subscribe | 2008-11-28 |
| ➤ Subscribe | Delayed-release Capsules | 45 mg | ➤ Subscribe | 2009-09-02 |
| ➤ Subscribe | Ophthalmic Solution | 0.5% | ➤ Subscribe | 2010-12-07 |
| ➤ Subscribe | Tablets | 12.5 mg, 25 mg, 50 mg, and 100 mg | ➤ Subscribe | 2013-01-14 |
| ➤ Subscribe | Capsules | 4 mcg | ➤ Subscribe | 2008-08-25 |
| ➤ Subscribe | Extended-release Capsules | 20 mg, 40 mg, 80 mg and 120 mg | ➤ Subscribe | 2017-07-25 |
| ➤ Subscribe | Capsules | 5 mg | ➤ Subscribe | 2005-08-17 |
| ➤ Subscribe | Tablets | 48 mg | ➤ Subscribe | 2008-07-01 |
| ➤ Subscribe | Gel | 10% | ➤ Subscribe | 2014-06-19 |
| ➤ Subscribe | Extended-release Capsules | 28 mg | ➤ Subscribe | 2013-06-12 |
| ➤ Subscribe | Injection | 10 mg/mL (2 mL) | ➤ Subscribe | 2018-07-13 |
| ➤ Subscribe | Tablets | 2.5 mg/200 mg | ➤ Subscribe | 2006-02-24 |
| ➤ Subscribe | Injection | 2 mg/mL, 10 mL vial | ➤ Subscribe | 2009-08-12 |
| ➤ Subscribe | Extended-release Capsules | 7 mg, 14 mg, 21 mg, and 28 mg | ➤ Subscribe | 2013-06-10 |
| ➤ Subscribe | Delayed-release Tablets | 800 mg | ➤ Subscribe | 2011-07-13 |
| ➤ Subscribe | Tablets | 10 mg, 20 mg, and 40 mg | ➤ Subscribe | 2015-01-21 |
| ➤ Subscribe | Ophthalmic Solution | 0.40% | ➤ Subscribe | 2005-01-28 |
| ➤ Subscribe | Capsules | 21 mg/10 mg | ➤ Subscribe | 2016-09-23 |
| ➤ Subscribe | Extended-release Tablets | 250 mg | ➤ Subscribe | 2004-05-03 |
| ➤ Subscribe | Opthalmic Solution | 0.45% | ➤ Subscribe | 2011-08-24 |
| ➤ Subscribe | Tablets | 100 mg/25 mg | ➤ Subscribe | 2008-12-23 |
| ➤ Subscribe | Extended-release Tablets | 7.5 mg and 15 mg | ➤ Subscribe | 2008-12-22 |
| ➤ Subscribe | Ophthalmic Solution | 0.15% | ➤ Subscribe | 2006-11-03 |
| ➤ Subscribe | Capsules | 145 mcg and 290 mcg | ➤ Subscribe | 2016-08-30 |
| ➤ Subscribe | Extended-release Tablets | 1 mg/240 mg | ➤ Subscribe | 2008-02-20 |
| ➤ Subscribe | Ophthalmic Solution | 0.25% | ➤ Subscribe | 2014-07-30 |
| ➤ Subscribe | Capsules | 100 mg | ➤ Subscribe | 2012-10-31 |
| ➤ Subscribe | Tablets | 5 mg/80 mg | ➤ Subscribe | 2017-06-09 |
| ➤ Subscribe | Ophthalmic Solution | 0.01% | ➤ Subscribe | 2011-04-05 |
| ➤ Subscribe | Delayed-release Capsules | 400 mg | ➤ Subscribe | 2014-06-17 |
| ➤ Subscribe | Tablets | 5 mg and 10 mg | ➤ Subscribe | 2007-10-16 |
| ➤ Subscribe | Capsules | 1 mcg and 2 mcg | ➤ Subscribe | 2008-10-14 |
| ➤ Subscribe | Delayed-release Capsules | 135 mg | ➤ Subscribe | 2009-09-01 |
| ➤ Subscribe | Topical Solution | 0.03% | ➤ Subscribe | 2010-05-03 |
| ➤ Subscribe | Tablets | 400 mg and 600 mg | ➤ Subscribe | 2010-05-10 |
| ➤ Subscribe | Gel | 7.5% | ➤ Subscribe | 2017-02-13 |
| ➤ Subscribe | Extended-release Capsules | 7 mg, 14 mg, 21 mg, and 28 mg | ➤ Subscribe | 2013-08-16 |
| ➤ Subscribe | Capsules | 10 mg and 20 mg | ➤ Subscribe | 2005-03-30 |
| ➤ Subscribe | Tablets | 145 mg | ➤ Subscribe | 2007-10-19 |
| ➤ Subscribe | Injection | 2 mg/mL, 5 mL vial and 10 mg/mL, 20 mL vial | ➤ Subscribe | 2009-08-04 |
| ➤ Subscribe | Extended-release Capsules | 7 mg, 14 mg, 21 mg | ➤ Subscribe | 2013-06-17 |
| ➤ Subscribe | Suppository | 1000 mg | ➤ Subscribe | 2013-05-24 |
| ➤ Subscribe | Tablets | 5 mg/200 mg | ➤ Subscribe | 2005-05-27 |
| ➤ Subscribe | Injection | 400 mg/vial and 600 mg/vial | ➤ Subscribe | 2014-10-29 |
| ➤ Subscribe | Capsules | 14 mg/10 mg and 28 mg/10 mg | ➤ Subscribe | 2015-05-18 |
| ➤ Subscribe | Extended-release Tablet | 500 mg | ➤ Subscribe | 2010-12-06 |
International Patents for ABBVIE Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Canada | 2753387 | ⤷ Try a Trial |
| Canada | 2942823 | ⤷ Try a Trial |
| European Patent Office | 1991205 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for ABBVIE Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 3141251 | 301099 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: A MEDICINAL PRODUCT CONSISTING OF A COMBINATION OF A FIRST DOSE PHARMACEUTICAL COMPOSITION AND A SECOND DOSE PHARMACEUTICAL COMPOSITION, THE FIRST DOSE PHARMACEUTICAL COMPOSITION CONSISTING OF THE ACTIVE INGREDIENTS POLYETHYLENE GLYCOL, SODIUM SULPHATE, SODIUM CHLORIDE AND POTASSIUM CHLORIDE AND THE SECOND DOSE PHARMACEUTICAL COMPOSITION CONSISTING OF THE ACTIVE INGREDIENTS POLYETHYLENE GLYCOL, ASCORBIC ACID, SODIUM ASCORBATE, SODIUM CHLORIDE AND POTASSIUM CHLORIDE; NATIONAL REGISTRATION NO/DATE: RVG 120195 20171114; FIRST REGISTRATION: IS IS/1/17/083/01 20171016 |
| 1725537 | CR 2017 00008 | Denmark | ⤷ Try a Trial | PRODUCT NAME: ELUXADOLIN ELLER EN FARMACEUTISK ACCEPTABEL ENANTIOMER, DIASTEREOMER, RACEMAT ELLER SALT DERAF; REG. NO/DATE: EU/1/16/1126 20160921 |
| 2673237 | LUC00111 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: SODIUM ZIRCONIUM CYCLOSILICATE; AUTHORISATION NUMBER AND DATE: EU/1/17/1173 20180326 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.