twyneo Drug Patent Profile
✉ Email this page to a colleague
When do Twyneo patents expire, and when can generic versions of Twyneo launch?
Twyneo is a drug marketed by Galderma Labs Lp and is included in one NDA. There are five patents protecting this drug.
This drug has forty-seven patent family members in thirteen countries.
The generic ingredient in TWYNEO is benzoyl peroxide; tretinoin. There are seventeen drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the benzoyl peroxide; tretinoin profile page.
DrugPatentWatch® Generic Entry Outlook for Twyneo
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be December 30, 2030. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
Summary for twyneo
| International Patents: | 47 |
| US Patents: | 5 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for twyneo |
| What excipients (inactive ingredients) are in twyneo? | twyneo excipients list |
| DailyMed Link: | twyneo at DailyMed |
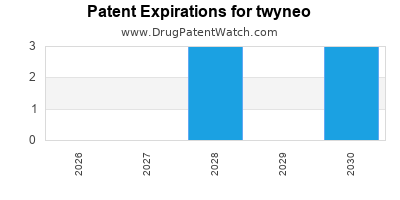
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for twyneo
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Pharmacology for twyneo
| Drug Class | Retinoid |
Anatomical Therapeutic Chemical (ATC) Classes for twyneo
US Patents and Regulatory Information for twyneo
twyneo is protected by five US patents and one FDA Regulatory Exclusivity.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of twyneo is ⤷ Sign Up.
This potential generic entry date is based on patent ⤷ Sign Up.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting twyneo
Methods and compositions for the treatment of acne
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TOPICAL TREATMENT OF ACNE VULGARIS IN ADULTS AND PEDIATRIC PATIENTS 9 YEARS OF AGE AND OLDER
Core stabilized microcapsules, method of their preparation and uses thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TOPICAL TREATMENT OF ACNE VULGARIS IN ADULTS AND PEDIATRIC PATIENTS 9 YEARS OF AGE AND OLDER
Core stabilized microcapsules, method of their preparation and uses thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Compositions for topical application comprising a peroxide and retinoid
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Metal oxide coating of water insoluble ingredients
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TOPICAL TREATMENT OF ACNE VULGARIS IN ADULTS AND PEDIATRIC PATIENTS 9 YEARS OF AGE AND OLDER
FDA Regulatory Exclusivity protecting twyneo
NEW COMBINATION
Exclusivity Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Galderma Labs Lp | TWYNEO | benzoyl peroxide; tretinoin | CREAM;TOPICAL | 214902-001 | Jul 26, 2021 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Galderma Labs Lp | TWYNEO | benzoyl peroxide; tretinoin | CREAM;TOPICAL | 214902-001 | Jul 26, 2021 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Galderma Labs Lp | TWYNEO | benzoyl peroxide; tretinoin | CREAM;TOPICAL | 214902-001 | Jul 26, 2021 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Galderma Labs Lp | TWYNEO | benzoyl peroxide; tretinoin | CREAM;TOPICAL | 214902-001 | Jul 26, 2021 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Galderma Labs Lp | TWYNEO | benzoyl peroxide; tretinoin | CREAM;TOPICAL | 214902-001 | Jul 26, 2021 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for twyneo
When does loss-of-exclusivity occur for twyneo?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 10337830
Estimated Expiration: ⤷ Sign Up
Brazil
Patent: 2012012023
Estimated Expiration: ⤷ Sign Up
Canada
Patent: 73544
Estimated Expiration: ⤷ Sign Up
China
Patent: 2596186
Estimated Expiration: ⤷ Sign Up
Eurasian Patent Organization
Patent: 1200347
Estimated Expiration: ⤷ Sign Up
European Patent Office
Patent: 67132
Estimated Expiration: ⤷ Sign Up
Japan
Patent: 34107
Estimated Expiration: ⤷ Sign Up
Patent: 13516404
Estimated Expiration: ⤷ Sign Up
Mexico
Patent: 4128
Estimated Expiration: ⤷ Sign Up
Patent: 12006636
Estimated Expiration: ⤷ Sign Up
South Africa
Patent: 1203582
Estimated Expiration: ⤷ Sign Up
South Korea
Patent: 130008002
Estimated Expiration: ⤷ Sign Up
Spain
Patent: 11499
Estimated Expiration: ⤷ Sign Up
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering twyneo around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Australia | 2006274541 | Metal oxide coating of water insoluble ingredients | ⤷ Sign Up |
| Japan | 5923065 | ⤷ Sign Up | |
| Eurasian Patent Organization | 200970724 | КОМПОЗИЦИИ ДЛЯ МЕСТНОГО ПРИМЕНЕНИЯ, СОДЕРЖАЩИЕ ПЕРОКСИД И РЕТИНОИД | ⤷ Sign Up |
| Mexico | 344128 | MICROCAPSULAS ESTABILIZADAS CON NUCLEO, METODO PARA SU PREPARACION Y USOS DE LAS MISMAS. (CORE STABILIZED MICROCAPSULES, METHOD OF THEIR PREPARATION AND USES THEREOF.) | ⤷ Sign Up |
| Japan | 2013213063 | COMPOSITION FOR TOPICAL APPLICATION COMPRISING PEROXIDE AND RETINOID | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for twyneo
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0137963 | 97C0042 | Belgium | ⤷ Sign Up | PRODUCT NAME: 2-(2-BENZOYL-SUBSTITUE)-1,3-CYCLOHEXANE-DIONES; REGISTRATION NO/DATE: 8452/B 19930121 |
| 1458369 | C01458369/01 | Switzerland | ⤷ Sign Up | PRODUCT NAME: ADAPALENUM + BENZOYLIS PEROXIDUM; REGISTRATION NUMBER/DATE: SWISSMEDIC 58460 19.05.2009 |
| 1458369 | CA 2008 00029 | Denmark | ⤷ Sign Up | PRODUCT NAME: ADAPALEN, BENZOYLPEROXID |
| 1667986 | 92172 | Luxembourg | ⤷ Sign Up | PRODUCT NAME: SOLVAT ACETONIQUE DU CABAZITAXEL, OU DESIGNE SOLVAT ACETONIQUE DU DIMETHOXY DOCETAXEL OU SOLVAT ACETONIQUE DU (2R,3S)-3-TERT-BUTOXYCARBONYLAMINO-2-HYDROXY-3-PHENYLPROPIONATE DE 4-ACETOXY-2A-BENZOYLOXY-5BETA,20-EPOXY-1-HYDROXY-7BETA,10A-DIMETHOXY-9-OXO-TAX-11-ENE-13A-YLE(ACETONATE DU CABAZITAXEL) |
| 0591275 | SPC/GB05/030 | United Kingdom | ⤷ Sign Up | PRODUCT NAME: NITISINONE (2-(2-NITRO-4-TRIFLUOROMETHYLBENZOYL)-1,3-CYCLOHEXANEDIONE) OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF; REGISTERED: UK EU/1/04/303/001 20050221; UK EU/1/04/303/002 20050221; UK EU/1/04/303/003 20050221 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


