kalydeco Drug Patent Profile
✉ Email this page to a colleague
When do Kalydeco patents expire, and when can generic versions of Kalydeco launch?
Kalydeco is a drug marketed by Vertex Pharms Inc and Vertex Pharms and is included in two NDAs. There are fourteen patents protecting this drug and two Paragraph IV challenges.
This drug has two hundred and fifty-seven patent family members in thirty-six countries.
The generic ingredient in KALYDECO is ivacaftor. There are three drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the ivacaftor profile page.
DrugPatentWatch® Generic Entry Outlook for Kalydeco
Kalydeco was eligible for patent challenges on January 31, 2016.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be February 13, 2030. This may change due to patent challenges or generic licensing.
There have been five patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
There are two tentative approvals for the generic drug (ivacaftor), which indicates the potential for near-term generic launch.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for kalydeco?
- What are the global sales for kalydeco?
- What is Average Wholesale Price for kalydeco?
Summary for kalydeco
| International Patents: | 257 |
| US Patents: | 14 |
| Applicants: | 2 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 94 |
| Clinical Trials: | 26 |
| Patent Applications: | 1,513 |
| Drug Prices: | Drug price information for kalydeco |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for kalydeco |
| What excipients (inactive ingredients) are in kalydeco? | kalydeco excipients list |
| DailyMed Link: | kalydeco at DailyMed |
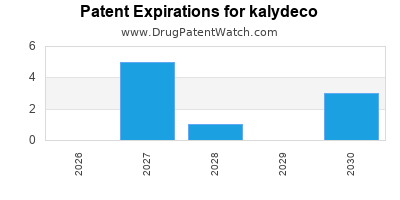

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for kalydeco
Generic Entry Dates for kalydeco*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
GRANULE;ORAL |
Generic Entry Dates for kalydeco*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for kalydeco
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) | Phase 2 |
| University of Miami | Early Phase 1 |
| University of Kansas Medical Center | Early Phase 1 |
Pharmacology for kalydeco
US Patents and Regulatory Information for kalydeco
kalydeco is protected by seventeen US patents and eleven FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of kalydeco is ⤷ Get Started Free.
This potential generic entry date is based on patent ⤷ Get Started Free.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Expired US Patents for kalydeco
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Vertex Pharms Inc | KALYDECO | ivacaftor | GRANULE;ORAL | 207925-001 | Mar 17, 2015 | ⤷ Get Started Free | ⤷ Get Started Free |
| Vertex Pharms Inc | KALYDECO | ivacaftor | GRANULE;ORAL | 207925-004 | May 3, 2023 | ⤷ Get Started Free | ⤷ Get Started Free |
| Vertex Pharms Inc | KALYDECO | ivacaftor | GRANULE;ORAL | 207925-005 | May 3, 2023 | ⤷ Get Started Free | ⤷ Get Started Free |
| Vertex Pharms Inc | KALYDECO | ivacaftor | GRANULE;ORAL | 207925-002 | Mar 17, 2015 | ⤷ Get Started Free | ⤷ Get Started Free |
| Vertex Pharms Inc | KALYDECO | ivacaftor | GRANULE;ORAL | 207925-003 | Apr 29, 2019 | ⤷ Get Started Free | ⤷ Get Started Free |
| Vertex Pharms | KALYDECO | ivacaftor | TABLET;ORAL | 203188-001 | Jan 31, 2012 | ⤷ Get Started Free | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for kalydeco
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Vertex Pharmaceuticals (Ireland) Limited | Kalydeco | ivacaftor | EMEA/H/C/002494Kalydeco tablets are indicated:As monotherapy for the treatment of adults, adolescents, and children aged 6 years and older and weighing 25 kg or more with cystic fibrosis (CF) who have an R117H CFTR mutation or one of the following gating (class III) mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene: G551D, G1244E, G1349D, G178R, G551S, S1251N, S1255P, S549N or S549R (see sections 4.4 and 5.1).In a combination regimen with tezacaftor/ivacaftor tablets for the treatment of adults, adolescents, and children aged 6 years and older with cystic fibrosis (CF) who are homozygous for the F508del mutation or who are heterozygous for the F508del mutation and have one of the following mutations in the CFTR gene: P67L, R117C, L206W, R352Q, A455E, D579G, 711+3A→G, S945L, S977F, R1070W, D1152H, 2789+5G→A, 3272 26A→G, and 3849+10kbC→T.In a combination regimen with ivacaftor/tezacaftor/elexacaftor tablets for the treatment of adults, adolescents, and children aged 6 years and older with cystic fibrosis (CF) who have at least one F508del mutation in the CFTR gene (see section 5.1).Kalydeco granules are indicated for the treatment of infants aged at least 4 months, toddlers and children weighing 5 kg to less than 25 kg with cystic fibrosis (CF) who have an R117H CFTR mutation or one of the following gating (class III) mutations in the CFTR gene: G551D, G1244E, G1349D, G178R, G551S, S1251N, S1255P, S549N or S549R (see sections 4.4 and 5.1). | Authorised | no | no | no | 2012-07-23 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for kalydeco
When does loss-of-exclusivity occur for kalydeco?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 09282419
Estimated Expiration: ⤷ Get Started Free
Patent: 10282986
Estimated Expiration: ⤷ Get Started Free
Patent: 13226076
Estimated Expiration: ⤷ Get Started Free
Patent: 16216569
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0916877
Estimated Expiration: ⤷ Get Started Free
Patent: 2012008082
Estimated Expiration: ⤷ Get Started Free
Patent: 2014021090
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 33908
Estimated Expiration: ⤷ Get Started Free
Patent: 69695
Estimated Expiration: ⤷ Get Started Free
Patent: 65519
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 12000348
Estimated Expiration: ⤷ Get Started Free
China
Patent: 2231990
Estimated Expiration: ⤷ Get Started Free
Patent: 2497859
Estimated Expiration: ⤷ Get Started Free
Patent: 4470518
Estimated Expiration: ⤷ Get Started Free
Patent: 9966264
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0180328
Estimated Expiration: ⤷ Get Started Free
Patent: 0190660
Estimated Expiration: ⤷ Get Started Free
Patent: 0210208
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 19945
Estimated Expiration: ⤷ Get Started Free
Patent: 21572
Estimated Expiration: ⤷ Get Started Free
Patent: 23901
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 28618
Estimated Expiration: ⤷ Get Started Free
Patent: 64337
Estimated Expiration: ⤷ Get Started Free
Patent: 45625
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 1706
Estimated Expiration: ⤷ Get Started Free
Patent: 1170330
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 28618
Estimated Expiration: ⤷ Get Started Free
Patent: 64337
Estimated Expiration: ⤷ Get Started Free
Patent: 19670
Estimated Expiration: ⤷ Get Started Free
Patent: 45625
Estimated Expiration: ⤷ Get Started Free
Patent: 42037
Estimated Expiration: ⤷ Get Started Free
Finland
Patent: 64337
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 61140
Estimated Expiration: ⤷ Get Started Free
Patent: 03840
Estimated Expiration: ⤷ Get Started Free
Patent: 05690
Estimated Expiration: ⤷ Get Started Free
Patent: 56805
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 35931
Estimated Expiration: ⤷ Get Started Free
Patent: 42437
Estimated Expiration: ⤷ Get Started Free
Patent: 53357
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 1203
Estimated Expiration: ⤷ Get Started Free
Patent: 4307
Estimated Expiration: ⤷ Get Started Free
Patent: 2421
Estimated Expiration: ⤷ Get Started Free
Patent: 5430
Estimated Expiration: ⤷ Get Started Free
Patent: 5854
Estimated Expiration: ⤷ Get Started Free
Patent: 1180
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 75768
Estimated Expiration: ⤷ Get Started Free
Patent: 34041
Estimated Expiration: ⤷ Get Started Free
Patent: 11530598
Estimated Expiration: ⤷ Get Started Free
Patent: 13501787
Estimated Expiration: ⤷ Get Started Free
Patent: 14111656
Estimated Expiration: ⤷ Get Started Free
Patent: 15511583
Estimated Expiration: ⤷ Get Started Free
Patent: 17190356
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 28618
Estimated Expiration: ⤷ Get Started Free
Patent: 64337
Estimated Expiration: ⤷ Get Started Free
Patent: 45625
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 6161
Estimated Expiration: ⤷ Get Started Free
Patent: 3230
Estimated Expiration: ⤷ Get Started Free
Patent: 9751
Estimated Expiration: ⤷ Get Started Free
Patent: 11001782
Estimated Expiration: ⤷ Get Started Free
Patent: 12001939
Estimated Expiration: ⤷ Get Started Free
Patent: 14010253
Estimated Expiration: ⤷ Get Started Free
Montenegro
Patent: 019
Estimated Expiration: ⤷ Get Started Free
Patent: 356
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 1535
Estimated Expiration: ⤷ Get Started Free
Patent: 7823
Estimated Expiration: ⤷ Get Started Free
Patent: 9199
Estimated Expiration: ⤷ Get Started Free
Norway
Patent: 28618
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 28618
Estimated Expiration: ⤷ Get Started Free
Patent: 64337
Estimated Expiration: ⤷ Get Started Free
Patent: 45625
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 28618
Estimated Expiration: ⤷ Get Started Free
Patent: 64337
Estimated Expiration: ⤷ Get Started Free
Patent: 45625
Estimated Expiration: ⤷ Get Started Free
Russian Federation
Patent: 92779
Estimated Expiration: ⤷ Get Started Free
Patent: 12109390
Estimated Expiration: ⤷ Get Started Free
Patent: 14139006
Estimated Expiration: ⤷ Get Started Free
Patent: 19116577
Estimated Expiration: ⤷ Get Started Free
San Marino
Patent: 01800074
Estimated Expiration: ⤷ Get Started Free
Patent: 01900210
Estimated Expiration: ⤷ Get Started Free
Patent: 02100077
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 894
Estimated Expiration: ⤷ Get Started Free
Patent: 604
Estimated Expiration: ⤷ Get Started Free
Patent: 408
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 8337
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 28618
Estimated Expiration: ⤷ Get Started Free
Patent: 64337
Estimated Expiration: ⤷ Get Started Free
Patent: 45625
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1101097
Estimated Expiration: ⤷ Get Started Free
Patent: 1200722
Estimated Expiration: ⤷ Get Started Free
Patent: 1406233
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 110042356
Estimated Expiration: ⤷ Get Started Free
Patent: 120061875
Estimated Expiration: ⤷ Get Started Free
Patent: 170072950
Estimated Expiration: ⤷ Get Started Free
Patent: 190143497
Estimated Expiration: ⤷ Get Started Free
Patent: 220057663
Estimated Expiration: ⤷ Get Started Free
Patent: 240066199
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 60143
Estimated Expiration: ⤷ Get Started Free
Patent: 18273
Estimated Expiration: ⤷ Get Started Free
Patent: 57152
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 2261
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering kalydeco around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Lithuania | 3219705 | ⤷ Get Started Free | |
| Portugal | 1773816 | ⤷ Get Started Free | |
| Canada | 2865519 | ⤷ Get Started Free | |
| Mexico | 2012001939 | ⤷ Get Started Free | |
| South Africa | 201200722 | ⤷ Get Started Free | |
| Russian Federation | 2019116577 | ⤷ Get Started Free | |
| Australia | 2016216569 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for kalydeco
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1773816 | 15C0045 | France | ⤷ Get Started Free | PRODUCT NAME: IVACAFTOR, OU L'UN DE SES SELS PHARMACEUTIQUEMENT ACCEPTABLES; REGISTRATION NO/DATE: EU/1/12/782/001-002 20120725 |
| 1773816 | CR 2015 00038 | Denmark | ⤷ Get Started Free | PRODUCT NAME: N-(5-HYDROXY-2,4-DITERT-BUTYL-PHENYL)-4-OXO-1H-QUINOLIN-3-CARBOXAMID ELLER ET FARMACEUTISK ACCEPTABELT SALT DERAF; REG. NO/DATE: EU/1/12/782/001-002 20120725 |
| 1773816 | CA 2015 00038 | Denmark | ⤷ Get Started Free | PRODUCT NAME: IVACAFTOR, N-(5-HYDROXY-2,4-DITERT-BUTYL-PHENYL)-4-OXO-1H-QUINOLIN-3-CARBOXAMID ELLER ET FARMACEUTISK ACCEPTABLET SALT DERAF; REG. NO/DATE: EU/1/12/782/001-002 20120723 |
| 3170818 | 132020000000103 | Italy | ⤷ Get Started Free | PRODUCT NAME: UNA COMBINAZIONE DI (A) LUMACAFTOR E (B) IVACAFTOR O UN SUO SALE FARMACEUTICAMENTE ACCETTABILE(ORKAMBI); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/15/1059, 20151124 |
| 1773816 | C01773816/01 | Switzerland | ⤷ Get Started Free | PRODUCT NAME: IVACAFTOR; REGISTRATION NO/DATE: SWISSMEDIC 62686 13.01.2014 |
| 1773816 | PA2015028 | Lithuania | ⤷ Get Started Free | PRODUCT NAME: IVACAFTORUM; REGISTRATION NO/DATE: EU/1/12/782/001 - EU/1/12/782/002 20120723 |
| 1773816 | 122015000050 | Germany | ⤷ Get Started Free | PRODUCT NAME: N-(5-HYDROXY-2,4-DI-TERT-BUTYL-PHENYL)-4-OXO-1H-CHINOLIN-3-CARBONSAEUREAMID ODER EIN PHARMAZEUTISCH AKZEPTABLES SALZ DAVON; REGISTRATION NO/DATE: EU/1/12/782/001-002 20120723 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for KALYDECO (ivacaftor)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
