ZYVOX Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Zyvox, and what generic alternatives are available?
Zyvox is a drug marketed by Pfizer and is included in three NDAs.
The generic ingredient in ZYVOX is linezolid. There are twenty-two drug master file entries for this compound. Twenty-four suppliers are listed for this compound. Additional details are available on the linezolid profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Zyvox
A generic version of ZYVOX was approved as linezolid by HIKMA on June 3rd, 2015.
Summary for ZYVOX
| US Patents: | 0 |
| Applicants: | 1 |
| NDAs: | 3 |
| Finished Product Suppliers / Packagers: | 3 |
| Raw Ingredient (Bulk) Api Vendors: | 121 |
| Patent Applications: | 5,041 |
| Formulation / Manufacturing: | see details |
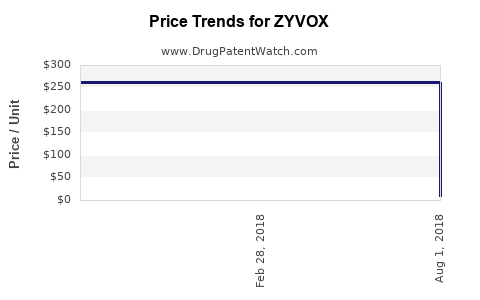
| Drug Prices: | Drug price information for ZYVOX |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for ZYVOX |
| What excipients (inactive ingredients) are in ZYVOX? | ZYVOX excipients list |
| DailyMed Link: | ZYVOX at DailyMed |
Pharmacology for ZYVOX
| Drug Class | Oxazolidinone Antibacterial |
Anatomical Therapeutic Chemical (ATC) Classes for ZYVOX
Paragraph IV (Patent) Challenges for ZYVOX
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| ZYVOX | Injection | linezolid | 2 mg/mL, 100 mL bag | 021131 | 1 | 2009-12-29 |
| ZYVOX | Injection | linezolid | 2 mg/mL, 300 mL bag | 021131 | 1 | 2009-09-01 |
| ZYVOX | Oral Suspension | linezolid | 100 mg/5 mL | 021132 | 1 | 2009-08-03 |
| ZYVOX | Tablets | linezolid | 600 mg | 021130 | 1 | 2005-12-21 |
US Patents and Regulatory Information for ZYVOX
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pfizer | ZYVOX | linezolid | FOR SUSPENSION;ORAL | 021132-001 | Apr 18, 2000 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Pfizer | ZYVOX | linezolid | SOLUTION;INTRAVENOUS | 021131-003 | Apr 18, 2000 | AP | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Pfizer | ZYVOX | linezolid | SOLUTION;INTRAVENOUS | 021131-001 | Apr 18, 2000 | AP | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Pfizer | ZYVOX | linezolid | SOLUTION;INTRAVENOUS | 021131-002 | Apr 18, 2000 | DISCN | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Pfizer | ZYVOX | linezolid | TABLET;ORAL | 021130-002 | Apr 18, 2000 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Pfizer | ZYVOX | linezolid | TABLET;ORAL | 021130-001 | Apr 18, 2000 | DISCN | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for ZYVOX
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Pfizer | ZYVOX | linezolid | TABLET;ORAL | 021130-002 | Apr 18, 2000 | ⤷ Sign Up | ⤷ Sign Up |
| Pfizer | ZYVOX | linezolid | SOLUTION;INTRAVENOUS | 021131-001 | Apr 18, 2000 | ⤷ Sign Up | ⤷ Sign Up |
| Pfizer | ZYVOX | linezolid | FOR SUSPENSION;ORAL | 021132-001 | Apr 18, 2000 | ⤷ Sign Up | ⤷ Sign Up |
| Pfizer | ZYVOX | linezolid | TABLET;ORAL | 021130-002 | Apr 18, 2000 | ⤷ Sign Up | ⤷ Sign Up |
| Pfizer | ZYVOX | linezolid | SOLUTION;INTRAVENOUS | 021131-003 | Apr 18, 2000 | ⤷ Sign Up | ⤷ Sign Up |
| Pfizer | ZYVOX | linezolid | TABLET;ORAL | 021130-002 | Apr 18, 2000 | ⤷ Sign Up | ⤷ Sign Up |
| Pfizer | ZYVOX | linezolid | TABLET;ORAL | 021130-001 | Apr 18, 2000 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for ZYVOX
See the table below for patents covering ZYVOX around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Taiwan | I293028 | ⤷ Sign Up | |
| New Zealand | 271805 | OXAZINE AND THIAZINE OXAZOLIDINONE DERIVATIVES AND ANTIMICROBIAL COMPOSITIONS THEREOF | ⤷ Sign Up |
| South Korea | 100864745 | ⤷ Sign Up | |
| Hong Kong | 1054325 | 噁唑烷酮片劑的配方 (OXAZOLIDINONE TABLET FORMULATION) | ⤷ Sign Up |
| Denmark | 1265608 | ⤷ Sign Up | |
| Canada | 2395603 | FACIES DE CRISTAUX DE LINEZOLIDE (FORME II) (LINEZOLID-CRYSTAL FORM II) | ⤷ Sign Up |
| China | 1130379 | ⤷ Sign Up | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for ZYVOX
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0717738 | 02C0005 | France | ⤷ Sign Up | PRODUCT NAME: LINEZOLIDE; NAT. REGISTRATION NO/DATE: NL 26 660 20010831; FIRST REGISTRATION: GB - PL 00 32/0261 20010105 |
| 0717738 | SPC001/2002 | Ireland | ⤷ Sign Up | SPC001/2002: 20031204, EXPIRES: 20160104 |
| 0717738 | CA 2002 00004 | Denmark | ⤷ Sign Up | |
| 0717738 | PA2004004,C0717738 | Lithuania | ⤷ Sign Up | PRODUCT NAME: LINEZOLIDUM ((S)-N-((3-(3-FLUOR-4-(4-MORFOLINIL)FENIL)-2-OKSO--5-OKSAZOLIDINIL)METIL)-ACETAMIDAS); REGISTRATION NO/DATE: 02/8199/2 20030313 |
| 0717738 | PA2004004 | Lithuania | ⤷ Sign Up | PRODUCTNAME: LINEZOLIDUM - (S)-N-[[3-FLUOR-4-(4-MORFOLINIL)FENIL]-2-OKSO-5-OKSAZOLIDINIL]METIL]-ACETAMIDAS |
| 0717738 | SPC/GB01/025 | United Kingdom | ⤷ Sign Up | PRODUCT NAME: LINEZOLID; REGISTERED: UK PL 00032/0259 20010105; UK PL 00032/0260 20010105; UK PL 00032/0261 20010105; UK PL 00032/0262 20010105 |
| 0717738 | 12/2002 | Austria | ⤷ Sign Up | PRODUCT NAME: LINEZOLID; NAT. REGISTRATION NO/DATE: 1-24227 - 1-24230 20011009; FIRST REGISTRATION: GB PL 00032/0259 - PL 00032/0262 20010105 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


