ZERBAXA Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Zerbaxa, and what generic alternatives are available?
Zerbaxa is a drug marketed by Cubist Pharms Llc and is included in one NDA. There are fourteen patents protecting this drug.
This drug has ninety-four patent family members in thirty-two countries.
The generic ingredient in ZERBAXA is ceftolozane sulfate; tazobactam sodium. One supplier is listed for this compound. Additional details are available on the ceftolozane sulfate; tazobactam sodium profile page.
DrugPatentWatch® Generic Entry Outlook for Zerbaxa
Zerbaxa was eligible for patent challenges on December 19, 2018.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be August 14, 2035. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
Summary for ZERBAXA
| International Patents: | 94 |
| US Patents: | 14 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 1 |
| Clinical Trials: | 6 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for ZERBAXA |
| What excipients (inactive ingredients) are in ZERBAXA? | ZERBAXA excipients list |
| DailyMed Link: | ZERBAXA at DailyMed |
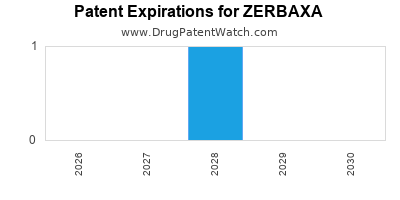

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for ZERBAXA
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for ZERBAXA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Hamad Medical Corporation | Phase 4 |
| University Hospital, Toulouse | Phase 3 |
| Royal Brisbane and Women's Hospital | Phase 1/Phase 2 |
Pharmacology for ZERBAXA
| Drug Class | Cephalosporin Antibacterial beta Lactamase Inhibitor |
| Mechanism of Action | beta Lactamase Inhibitors |
Anatomical Therapeutic Chemical (ATC) Classes for ZERBAXA
US Patents and Regulatory Information for ZERBAXA
ZERBAXA is protected by twenty-eight US patents and three FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of ZERBAXA is ⤷ Sign Up.
This potential generic entry date is based on patent ⤷ Sign Up.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting ZERBAXA
Methods for treating intrapulmonary infections
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF VENTILATOR-ASSOCIATED BACTERIAL PNEUMONIA
Methods for treating intrapulmonary infections
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF HOSPITAL-ACQUIRED BACTERIAL PNEUMONIA
Synthesis of cephalosporin compounds
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Treating infections with ceftolozane/tazobactam in subjects having impaired renal function
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF COMPLICATED URINARY TRACT INFECTION IN PATIENTS WITH END-STAGE RENAL DISEASE ON HEMODIALYSIS
Treating infections with ceftolozane/tazobactam in subjects having impaired renal function
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF COMPLICATED INTRA-ABDOMINAL INFECTION IN PATIENTS WITH END-STAGE RENAL DISEASE ON HEMODIALYSIS
Ceftolozane antibiotic compositions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF COMPLICATED URINARY TRACT INFECTION
Ceftolozane antibiotic compositions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF COMPLICATED INTRA-ABDOMINAL INFECTION
Ceftolozane antibiotic compositions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: COMPLICATED URINARY TRACT INFECTIONS (CUTI), INCLUDING PYELONEPHRITIS, IN ADULT AND PEDIATRIC PATIENTS (BIRTH TO LESS THAN 18 YEARS OLD)
Ceftolozane antibiotic compositions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: COMPLICATED INTRA-ABDOMINAL INFECTIONS (CIAI), USED IN COMBINATION WITH METRONIDAZOLE, IN ADULT AND PEDIATRIC PATIENTS (BIRTH TO LESS THAN 18 YEARS OF AGE)
Treating infections with ceftolozane/tazobactam in subjects having impaired renal function
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF VENTILATOR-ASSOCIATED BACTERIAL PNEUMONIA IN PATIENTS WITH END-STAGE RENAL DISEASE ON HEMODIALYSIS
Treating infections with ceftolozane/tazobactam in subjects having impaired renal function
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF HOSPITAL-ACQUIRED BACTERIAL PNEUMONIA IN PATIENTS WITH END-STAGE RENAL DISEASE ON HEMODIALYSIS
Ceftolozane antibiotic compositions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF HOSPITAL-ACQUIRED BACTERIAL PNEUMONIA (HABP)
Ceftolozane antibiotic compositions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF VENTILATOR-ASSOCIATED BACTERIAL PNEUMONIA (VABP)
Cephem compounds
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: METHODS OF TREATING BACTERIAL ILLNESSES
Cephem compounds
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: METHODS FOR TREATING BACTERIAL INFECTIONS
Cephem compounds
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: COMPLICATED URINARY TRACT INFECTIONS (CUTI), INCLUDING PYELONEPHRITIS, IN ADULT AND PEDIATRIC PATIENTS (BIRTH TO LESS THAN 18 YEARS OLD)
Cephem compounds
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: COMPLICATED INTRA-ABDOMINAL INFECTIONS (CIAI), USED IN COMBINATION WITH METRONIDAZOLE, IN ADULT AND PEDIATRIC PATIENTS (BIRTH TO LESS THAN 18 YEARS OF AGE)
Tazobactam arginine compositions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Tazobactam arginine compositions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: METHODS OF TREATING BACTERIAL ILLNESSES
Solid forms of ceftolozane
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Ceftolozane-tazobactam pharmaceutical compositions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF COMPLICATED URINARY TRACT INFECTION, INCLUDING PYELONEPHRITIS
Ceftolozane-tazobactam pharmaceutical compositions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF COMPLICATED INTRA-ABDOMINAL INFECTION
Ceftolozane-tazobactam pharmaceutical compositions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: COMPLICATED URINARY TRACT INFECTIONS (CUTI), INCLUDING PYELONEPHRITIS, IN ADULT AND PEDIATRIC PATIENTS (BIRTH TO LESS THAN 18 YEARS OLD)
Ceftolozane-tazobactam pharmaceutical compositions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: COMPLICATED INTRA-ABDOMINAL INFECTIONS (CIAI), USED IN COMBINATION WITH METRONIDAZOLE, IN ADULT AND PEDIATRIC PATIENTS (BIRTH TO LESS THAN 18 YEARS OF AGE)
Ceftolozane-tazobactam pharmaceutical compositions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Methods for treating intrapulmonary infections
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF HOSPITAL-ACQUIRED BACTERIAL PNEUMONIA
Methods for treating intrapulmonary infections
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF VENTILATOR-ASSOCIATED BACTERIAL PNEUMONIA
Ceftolozane antibiotic compositions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
FDA Regulatory Exclusivity protecting ZERBAXA
NEW CHEMICAL ENTITY
Exclusivity Expiration: ⤷ Sign Up
NEW PATIENT POPULATION
Exclusivity Expiration: ⤷ Sign Up
GENERATING ANTIBIOTIC INCENTIVES NOW
Exclusivity Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cubist Pharms Llc | ZERBAXA | ceftolozane sulfate; tazobactam sodium | POWDER;INTRAVENOUS | 206829-001 | Dec 19, 2014 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Cubist Pharms Llc | ZERBAXA | ceftolozane sulfate; tazobactam sodium | POWDER;INTRAVENOUS | 206829-001 | Dec 19, 2014 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Cubist Pharms Llc | ZERBAXA | ceftolozane sulfate; tazobactam sodium | POWDER;INTRAVENOUS | 206829-001 | Dec 19, 2014 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| Cubist Pharms Llc | ZERBAXA | ceftolozane sulfate; tazobactam sodium | POWDER;INTRAVENOUS | 206829-001 | Dec 19, 2014 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Cubist Pharms Llc | ZERBAXA | ceftolozane sulfate; tazobactam sodium | POWDER;INTRAVENOUS | 206829-001 | Dec 19, 2014 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for ZERBAXA
When does loss-of-exclusivity occur for ZERBAXA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
China
Patent: 6795175
Estimated Expiration: ⤷ Sign Up
Patent: 0204558
Estimated Expiration: ⤷ Sign Up
European Patent Office
Patent: 80347
Estimated Expiration: ⤷ Sign Up
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering ZERBAXA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Russian Federation | 2703457 | Твёрдые формы цефтолозана (SOLID FORMS OF CEFTOLOZANE) | ⤷ Sign Up |
| South Korea | 101023035 | ⤷ Sign Up | |
| Australia | 2017279686 | Treating Infections with Ceftolozane/Tazobactam in Subjects Having Impaired Renal Function | ⤷ Sign Up |
| Japan | 6870029 | ⤷ Sign Up | |
| South Africa | 200504181 | Cephem compounds | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for ZERBAXA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1556389 | 6/2016 | Austria | ⤷ Sign Up | PRODUCT NAME: CEFTOLOZAN ODER EIN PHARMAZEUTISCH ANNEHMBARES SALZ DAVON, INSBESONDERE EIN SCHWEFELSAEURESALZ; REGISTRATION NO/DATE: EU/1/15/1032/001 (MITTEILUNG) 20150922 |
| 1556389 | 92943 | Luxembourg | ⤷ Sign Up | PRODUCT NAME: CEFTOLOZANE OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE, EN PARTICULIER UN SEL D'ACIDE SULFURIQUE (ZERBAXA); FIRST REGISTRATION: 20150922 |
| 1556389 | CA 2016 00004 | Denmark | ⤷ Sign Up | PRODUCT NAME: CEFTOLOZAN ELLER ET FARMACEUTISK ACCEPTABELT SALT DERAF, SAERLIGT ET SVOVLSYRESALT; REG. NO/DATE: EU/1/15/1032/001 20150922 |
| 1556389 | C01556389/01 | Switzerland | ⤷ Sign Up | PRODUCT NAME: CEFTOLOZAN; REGISTRATION NO/DATE: SWISSMEDIC-ZULASSUNG 65472 03.03.2016 |
| 1556389 | SPC/GB16/002 | United Kingdom | ⤷ Sign Up | PRODUCT NAME: CEFTOLOZANE OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF IN PARTICULAR A SULFURIC ACID SALT.; REGISTERED: UK EU/1/15/1032 20150922 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


