ZEJULA Drug Patent Profile
✉ Email this page to a colleague
When do Zejula patents expire, and when can generic versions of Zejula launch?
Zejula is a drug marketed by Glaxosmithkline and is included in two NDAs. There are eight patents protecting this drug.
This drug has two hundred and eighty patent family members in fifty-five countries.
The generic ingredient in ZEJULA is niraparib tosylate. One supplier is listed for this compound. Additional details are available on the niraparib tosylate profile page.
DrugPatentWatch® Generic Entry Outlook for Zejula
Zejula was eligible for patent challenges on March 27, 2021.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be March 27, 2038. This may change due to patent challenges or generic licensing.
There has been one patent litigation case involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
Summary for ZEJULA
| International Patents: | 280 |
| US Patents: | 8 |
| Applicants: | 1 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 68 |
| Clinical Trials: | 57 |
| Drug Prices: | Drug price information for ZEJULA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for ZEJULA |
| What excipients (inactive ingredients) are in ZEJULA? | ZEJULA excipients list |
| DailyMed Link: | ZEJULA at DailyMed |
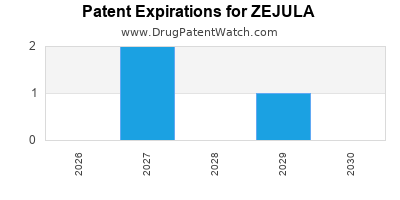

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for ZEJULA
Generic Entry Dates for ZEJULA*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
CAPSULE;ORAL |
Generic Entry Dates for ZEJULA*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for ZEJULA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| University of California, San Francisco | Phase 1 |
| Servier | Phase 3 |
| GlaxoSmithKline Research & Development Limited | Phase 3 |
Pharmacology for ZEJULA
| Drug Class | Poly(ADP-Ribose) Polymerase Inhibitor |
| Mechanism of Action | Poly(ADP-Ribose) Polymerase Inhibitors |
Anatomical Therapeutic Chemical (ATC) Classes for ZEJULA
US Patents and Regulatory Information for ZEJULA
ZEJULA is protected by nine US patents and three FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of ZEJULA is ⤷ Try a Trial.
This potential generic entry date is based on patent ⤷ Try a Trial.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting ZEJULA
Niraparib compositions
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: A METHOD OF TREATMENT OF OVARIAN CANCER OR FALLOPIAN TUBE CANCER
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: A METHOD OF TREATMENT OF RECURRENT OVARIAN CANCER OR FALLOPIAN TUBE CANCER ASSOCIATED WITH DELETERIOUS OR SUSPECTED DELETERIOUS GERMLINE BRCA-MUTATION
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
DNA damage repair inhibitors for the treatment of cancer
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: A METHOD OF TREATMENT OF ADVANCED OVARIAN, FALLOPIAN TUBE, OR PRIMARY PERITONEAL CANCER ASSOCIATED WITH HOMOLOGOUS RECOMBINATION DEFICIENCY (HRD) POSITIVE STATUS
Amide substituted indazoles as poly(ADP-ribose)polymerase(PARP) inhibitors
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
DNA damage repair inhibitors for treatment of cancer
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: A METHOD OF TREATMENT OF ADVANCED OVARIAN, FALLOPIAN TUBE, OR PRIMARY PERITONEAL CANCER ASSOCIATED WITH HOMOLOGOUS RECOMBINATION DEFICIENCY (HRD) POSITIVE STATUS
Pharmaceutically acceptable salts of 2-{4-[(3S)-piperidin-3-yl]phenyl}-2H-indazole-7-carboxamide
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Use of RNAI inhibiting PARP activity for the manufacture of a medicament for the treatment of cancer
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: A METHOD OF TREATMENT OF ADVANCED OVARIAN, FALLOPIAN TUBE, OR PRIMARY PERITONEAL CANCER ASSOCIATED WITH HOMOLOGOUS RECOMBINATION DEFICIENCY (HRD) POSITIVE STATUS
FDA Regulatory Exclusivity protecting ZEJULA
INDICATED FOR MAINTENANCE TREATMENT OF ADULT PATIENTS WITH RECURRENT EPITHELIAL OVARIAN, FALLOPIAN TUBE, OR PRIMARY PERITONEAL CANCER WHO ARE IN A COMPLETE OR PARTIAL RESPONSE TO PLATINUM-BASED CHEMOTHERAPY
Exclusivity Expiration: ⤷ Try a Trial
TX OF ADULTS W/ ADV OVARIAN, FALLOPIAN TUBE, OR PRIMARY PERITONEAL CANCER TREATED W/ >=3 PRIOR CHEMO REGIMENS & CANCER ASSOCIATED W/ HRD+ STATUS DEFINED BY A DELETERIOUS OR SUSPECTED DELETERIOUS BRCA MUTATION
Exclusivity Expiration: ⤷ Try a Trial
INDICATED FOR THE MAINTENANCE TREATMENT OF ADULT PATIENTS WITH ADVANCED EPITHELIAL OVARIAN CANCER WHO ARE IN A COMPLETE OR PARTIAL RESPONSE TO FIRST-LINE PLATINUM-BASED CHEMOTHERAPY
Exclusivity Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glaxosmithkline | ZEJULA | niraparib tosylate | TABLET;ORAL | 214876-003 | Apr 26, 2023 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Glaxosmithkline | ZEJULA | niraparib tosylate | TABLET;ORAL | 214876-002 | Apr 26, 2023 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Glaxosmithkline | ZEJULA | niraparib tosylate | TABLET;ORAL | 214876-003 | Apr 26, 2023 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Glaxosmithkline | ZEJULA | niraparib tosylate | TABLET;ORAL | 214876-002 | Apr 26, 2023 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for ZEJULA
When does loss-of-exclusivity occur for ZEJULA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 18246214
Estimated Expiration: ⤷ Try a Trial
Patent: 21245223
Estimated Expiration: ⤷ Try a Trial
Brazil
Patent: 2019020211
Estimated Expiration: ⤷ Try a Trial
Canada
Patent: 58375
Estimated Expiration: ⤷ Try a Trial
China
Patent: 0944638
Estimated Expiration: ⤷ Try a Trial
Eurasian Patent Organization
Patent: 1992177
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 00314
Estimated Expiration: ⤷ Try a Trial
Israel
Patent: 9630
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 20512350
Estimated Expiration: ⤷ Try a Trial
Mexico
Patent: 19011496
Estimated Expiration: ⤷ Try a Trial
Singapore
Patent: 201909011P
Estimated Expiration: ⤷ Try a Trial
South Korea
Patent: 200014736
Estimated Expiration: ⤷ Try a Trial
Taiwan
Patent: 61476
Estimated Expiration: ⤷ Try a Trial
Patent: 1840315
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering ZEJULA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Japan | 2007533601 | ⤷ Try a Trial | |
| European Patent Office | 1633724 | DERIVES DE PHTALAZINONE (PHTHALAZINONE DERIVATIVES) | ⤷ Try a Trial |
| Tunisia | 2009000286 | AMIDE SUBSTITUTED INDAZOLES AS POLY (ADP-RIBOSE)POLYMERASE (PARP) INHIBITORS | ⤷ Try a Trial |
| Spain | 2728199 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for ZEJULA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2109608 | C20180013 00262 | Estonia | ⤷ Try a Trial | PRODUCT NAME: NIRAPARIIB;REG NO/DATE: EU/1/17/1235 20.11.2017 |
| 2109608 | SPC/GB18/016 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: NIRAPARIB, OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF, PARTICULARLY THE TOSYLATE OR A HYDRATE, ESPECIALLY THE TOSYLATE MONOHYDRATE; REGISTERED: UK EU/1/17/1235 20171120; UK PLGB 19494/0294 20171120 |
| 2240466 | SPC/GB18/018 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: NIRAPARIB TOSYLATE OR A HYDRATE THEREOF, ESPECIALLY THE TOSYLATE MONOHYDRATE; REGISTERED: UK EU/1/17/1235 20171120; UK PLGB 19494/0294 20171120 |
| 1633724 | C300726 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: OLAPARIB, EN ZOUTEN EN; REGISTRATION NO/DATE: EU/1/14/959/001 20141216 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


