VYTORIN Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Vytorin, and when can generic versions of Vytorin launch?
Vytorin is a drug marketed by Organon and is included in one NDA.
The generic ingredient in VYTORIN is ezetimibe; simvastatin. There are twenty-four drug master file entries for this compound. Thirteen suppliers are listed for this compound. Additional details are available on the ezetimibe; simvastatin profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Vytorin
A generic version of VYTORIN was approved as ezetimibe; simvastatin by DR REDDYS LABS SA on April 26th, 2017.
Summary for VYTORIN
| US Patents: | 0 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 4 |
| Clinical Trials: | 41 |
| Patent Applications: | 206 |
| Formulation / Manufacturing: | see details |
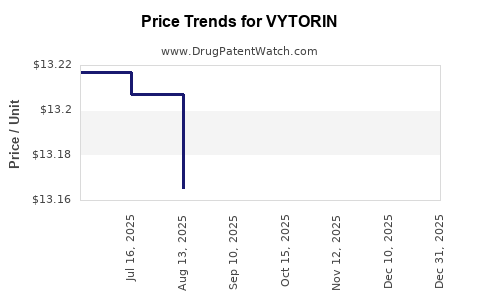
| Drug Prices: | Drug price information for VYTORIN |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for VYTORIN |
| What excipients (inactive ingredients) are in VYTORIN? | VYTORIN excipients list |
| DailyMed Link: | VYTORIN at DailyMed |
Recent Clinical Trials for VYTORIN
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Samsung Medical Center | Phase 4 |
| Cairo University | N/A |
| Aswan Heart Centre | N/A |
Pharmacology for VYTORIN
| Drug Class | Dietary Cholesterol Absorption Inhibitor HMG-CoA Reductase Inhibitor |
| Mechanism of Action | Hydroxymethylglutaryl-CoA Reductase Inhibitors |
| Physiological Effect | Decreased Cholesterol Absorption |
Anatomical Therapeutic Chemical (ATC) Classes for VYTORIN
Paragraph IV (Patent) Challenges for VYTORIN
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| VYTORIN | Tablets | ezetimibe; simvastatin | 10 mg/10 mg 10 mg/20 mg 10 mg/40 mg 10 mg/80 mg | 021687 | 1 | 2009-07-27 |
US Patents and Regulatory Information for VYTORIN
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Organon | VYTORIN | ezetimibe; simvastatin | TABLET;ORAL | 021687-001 | Jul 23, 2004 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Organon | VYTORIN | ezetimibe; simvastatin | TABLET;ORAL | 021687-004 | Jul 23, 2004 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Organon | VYTORIN | ezetimibe; simvastatin | TABLET;ORAL | 021687-002 | Jul 23, 2004 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Organon | VYTORIN | ezetimibe; simvastatin | TABLET;ORAL | 021687-003 | Jul 23, 2004 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for VYTORIN
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Organon | VYTORIN | ezetimibe; simvastatin | TABLET;ORAL | 021687-004 | Jul 23, 2004 | ⤷ Sign Up | ⤷ Sign Up |
| Organon | VYTORIN | ezetimibe; simvastatin | TABLET;ORAL | 021687-004 | Jul 23, 2004 | ⤷ Sign Up | ⤷ Sign Up |
| Organon | VYTORIN | ezetimibe; simvastatin | TABLET;ORAL | 021687-003 | Jul 23, 2004 | ⤷ Sign Up | ⤷ Sign Up |
| Organon | VYTORIN | ezetimibe; simvastatin | TABLET;ORAL | 021687-001 | Jul 23, 2004 | ⤷ Sign Up | ⤷ Sign Up |
| Organon | VYTORIN | ezetimibe; simvastatin | TABLET;ORAL | 021687-002 | Jul 23, 2004 | ⤷ Sign Up | ⤷ Sign Up |
| Organon | VYTORIN | ezetimibe; simvastatin | TABLET;ORAL | 021687-001 | Jul 23, 2004 | ⤷ Sign Up | ⤷ Sign Up |
| Organon | VYTORIN | ezetimibe; simvastatin | TABLET;ORAL | 021687-003 | Jul 23, 2004 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for VYTORIN
See the table below for patents covering VYTORIN around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| New Zealand | 200588 | 6(R)-(2-(8(S)-(2,2-DIMETHYLBUTYRYLOXY)-2(S),6(S)-DIMETHYL-1,2,3,4,4A(S),5,6,7,8,8A(S)-DECAHYDRONAPHTHYL-1(S))-ETHYL)-4(R)-HYDROXY-3,4,5,6-TETRAHYDRO-2H-PYRAN-2-ONE AND PHARMACEUTICAL COMPOSITIONS | ⤷ Sign Up |
| Malaysia | 8700745 | 6(R)-(2-8-ACYLOXY-2'-METHYL-6'METHYL(OR HYDROGEN)-POLYHYDRONAPTHYL-1')-ETHYL)-4(R)-HYDROXY-3,4,5,6-TETRAHYDRO-24-PYRAN-2-ONES, THE HYDROXY ACID FROM OF SAID PYRANONES, THE PARMACEUTICALLY ACCEPTABLE SATLS OF SAID HYDROXY ACIDS, AND THE LOWER ALKYL, AND PHENYL, DIMETHYLAMINO OR ACETYLAMINO SUBSTITUTED LOWER ALKYL ESTERS OF SAID HYDROXY ACID, PROCESSES FOR PREPARING THE SAME, AND A PARMACEUTICAL ANTIHYPERCHOLESTEROLEMIC COMPOSITION CONTAINING THE SAME. | ⤷ Sign Up |
| Slovakia | 281067 | ⤷ Sign Up | |
| China | 1310643 | ⤷ Sign Up | |
| Croatia | P930775 | ⤷ Sign Up | |
| Israel | 62044 | POLYHYDRONAPHTHYLETHYL TETRAHYDRO-2H-PYRAN-2-ONE DERIVATIVES AND HYDROXY ACIDS AND ESTERS THEREOF,THEIR PREPARATION AND PHARMACEUTICAL COMPOSITIONS CONTAINING THEM | ⤷ Sign Up |
| Malaysia | 148689 | PHARMACEUTICAL FORMULATION | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for VYTORIN
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0720599 | CR 2014 00048 | Denmark | ⤷ Sign Up | PRODUCT NAME: KOMBINATION AF ROSUVASTATIN OG EZETIMIBE ELLER FARMACEUTISK ACCEPTABLE SALTE DERAF, HERUNDER ROSUVASTATIN SOM ZINK; NAT. REG. NO/DATE: 52921, 52922, 52923 20140820; FIRST REG. NO/DATE: NO 13-9663, 13-9664, 13-9665 20140724 |
| 0720599 | 10399001 | Germany | ⤷ Sign Up | PRODUCT NAME: EZETIMIB ODER EIN PHARMAZEUTISCH ANNEHMBARES SALZ HIERVON; REGISTRATION NO/DATE: 54486.00.00 20021017 |
| 0720599 | 122004000026 | Germany | ⤷ Sign Up | PRODUCT NAME: EZETIMIB ODER PHARMAZEUTISCH ANNEHMBARE SALZE DAVON IN KOMBINATION MIT SIMVASTATIN; NAT. REGISTRATION NO/DATE: 58866.00.00 58866.01.00 58866.02.00 58866.03.00 58870.00.00 58870.01.00 58870.02.00 58870.03.00 58874.00.00 58874.01.00 58874.02.00 58874.03.00 58878.00.00 58878.01.00 58878.02.00 58878.03.00 20040402 FIRST REGISTRATION: DE 58866.00.00 - 58866.03.00 58870.00.00 - 58870.03.00 58874.00.00 - 58874.03.00 58878.00.00 - 58878.03.00 20040402 |
| 0720599 | 92545 | Luxembourg | ⤷ Sign Up | PRODUCT NAME: EZETIMIBE EN COMBINAISON AVEC ATORVASTATINE OU LEURS SELS PHARMACEUTIQUEMENT ACCEPTEES, Y COMPRIS ATORVASTATINE SOUS FORME D'ATORVASTATINE CALCIQUE TRIHYDRATEE; FIRST REGISTRATION: 20140910 |
| 0720599 | CR 2014 00050 | Denmark | ⤷ Sign Up | PRODUCT NAME: EZETIMIBE AND ATORVASTATIN OR PHARMACEUTICALLY ACCEPTABLE SALTS THEREOF, INCLUDING ATORVASTATIN AS ATORVASTATIN CALCIUM TRIHYDRATE; REG. NO/DATE: DE/H/3895-3898/001-004/DC 20140910 |
| 0720599 | CA 2005 00003 | Denmark | ⤷ Sign Up | |
| 0720599 | 0390018-0 | Sweden | ⤷ Sign Up | PRODUCT NAME: EZETIMIB |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


