VUITY Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Vuity, and when can generic versions of Vuity launch?
Vuity is a drug marketed by Abbvie and is included in one NDA. There are two patents protecting this drug and one Paragraph IV challenge.
This drug has thirty-one patent family members in twenty-three countries.
The generic ingredient in VUITY is pilocarpine hydrochloride. There are twelve drug master file entries for this compound. Fourteen suppliers are listed for this compound. Additional details are available on the pilocarpine hydrochloride profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Vuity
A generic version of VUITY was approved as pilocarpine hydrochloride by PADAGIS US on November 16th, 2004.
Summary for VUITY
| International Patents: | 31 |
| US Patents: | 2 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 75 |
| Patent Applications: | 3,839 |
| Formulation / Manufacturing: | see details |
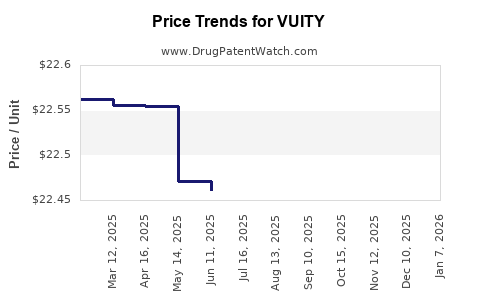
| Drug Prices: | Drug price information for VUITY |
| What excipients (inactive ingredients) are in VUITY? | VUITY excipients list |
| DailyMed Link: | VUITY at DailyMed |
Pharmacology for VUITY
| Drug Class | Cholinergic Receptor Agonist |
| Mechanism of Action | Cholinergic Agonists Cholinergic Muscarinic Agonists |
Anatomical Therapeutic Chemical (ATC) Classes for VUITY
Paragraph IV (Patent) Challenges for VUITY
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| VUITY | Ophthalmic Solution | pilocarpine hydrochloride | 1.25% | 214028 | 1 | 2022-12-30 |
US Patents and Regulatory Information for VUITY
VUITY is protected by six US patents and two FDA Regulatory Exclusivities.
Patents protecting VUITY
Presbyopia treatments
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF PRESBYOPIA IN ADULTS BY ADMINISTRATION OF PILOCARPINE HCI FORMULATION TWICE DAILY
Presbyopia treatments
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF PRESBYOPIA IN ADULTS BY ADMINISTRATION OF PILOCARPINE HCI FORMULATION ONCE DAILY
Presbyopia treatments
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: USE OF VUITY FOR THE TREATMENT OF PRESBYOPIA IN ADULTS
Presbyopia treatments
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF PRESBYOPIA IN ADULTS BY ADMINISTRATION OF PILOCARPINE HCI FORMULATION ONCE DAILY
Presbyopia treatments
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF PRESBYOPIA IN ADULTS BY ADMINISTRATION OF PILOCARPINE HCI FORMULATION TWICE DAILY
Presbyopia treatments
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: USE OF VUITY FOR THE TREATMENT OF PRESBYOPIA IN ADULTS
FDA Regulatory Exclusivity protecting VUITY
ADDITION OF SECOND DOSE FOR TREATMENT OF PRESBYOPIA IN ADULTS
Exclusivity Expiration: ⤷ Sign Up
NEW PRODUCT
Exclusivity Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbvie | VUITY | pilocarpine hydrochloride | SOLUTION;OPHTHALMIC | 214028-001 | Oct 28, 2021 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Abbvie | VUITY | pilocarpine hydrochloride | SOLUTION;OPHTHALMIC | 214028-001 | Oct 28, 2021 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Abbvie | VUITY | pilocarpine hydrochloride | SOLUTION;OPHTHALMIC | 214028-001 | Oct 28, 2021 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Abbvie | VUITY | pilocarpine hydrochloride | SOLUTION;OPHTHALMIC | 214028-001 | Oct 28, 2021 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for VUITY
See the table below for patents covering VUITY around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Japan | 2021522256 | 眼の状態の治療のためのピロカルピン塩酸塩の使用 | ⤷ Sign Up |
| South Korea | 20230079489 | 안구 증상의 치료를 위한 필로카르핀 염산염의 용도 (USE OF PILOCARPINE HYDROCHLORIDE FOR THE TREATMENT OF OCULAR CONDITIONS) | ⤷ Sign Up |
| Portugal | 3681500 | ⤷ Sign Up | |
| Australia | 2020203311 | Use of pilocarpine hydrochloride for the treatment of ocular conditions | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |


