VICTOZA Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Victoza, and what generic alternatives are available?
Victoza is a drug marketed by Novo Nordisk Inc and is included in one NDA. There are four patents protecting this drug and one Paragraph IV challenge.
This drug has one hundred patent family members in twenty-nine countries.
The generic ingredient in VICTOZA is liraglutide recombinant. There are seven drug master file entries for this compound. Two suppliers are listed for this compound. Additional details are available on the liraglutide recombinant profile page.
DrugPatentWatch® Generic Entry Outlook for Victoza
Victoza was eligible for patent challenges on January 25, 2014.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be February 13, 2026. This may change due to patent challenges or generic licensing.
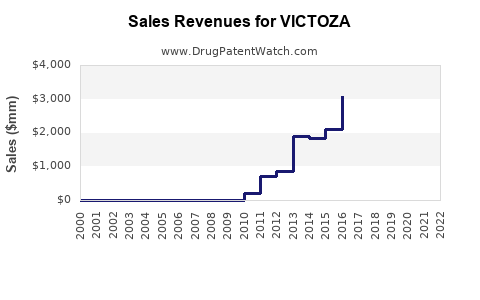
Annual sales in 2021 were $3.4bn, indicating a strong incentive for generic entry (peak sales were $5.6bn in 2019).
There have been nineteen patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
Summary for VICTOZA
| International Patents: | 100 |
| US Patents: | 4 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 27 |
| Clinical Trials: | 125 |
| Patent Applications: | 3,742 |
| Formulation / Manufacturing: | see details |
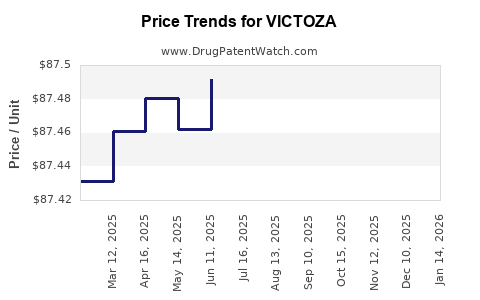
| Drug Prices: | Drug price information for VICTOZA |
| Drug Sales Revenues: | Drug sales revenues for VICTOZA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for VICTOZA |
| What excipients (inactive ingredients) are in VICTOZA? | VICTOZA excipients list |
| DailyMed Link: | VICTOZA at DailyMed |
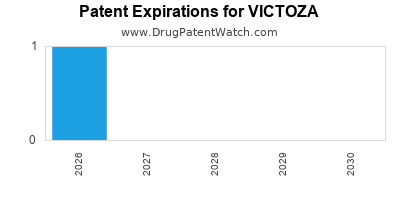
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for VICTOZA
Generic Entry Date for VICTOZA*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
SOLUTION;SUBCUTANEOUS |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for VICTOZA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Carmot Therapeutics, Inc. | Phase 1 |
| Yale University | Phase 3 |
| Biolingus | Phase 1/Phase 2 |
Pharmacology for VICTOZA
| Drug Class | GLP-1 Receptor Agonist |
| Mechanism of Action | Glucagon-like Peptide-1 (GLP-1) Agonists |
Anatomical Therapeutic Chemical (ATC) Classes for VICTOZA
Paragraph IV (Patent) Challenges for VICTOZA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| VICTOZA | Injection | liraglutide recombinant | 18 mg/3 mL prefilled syringe | 022341 | 1 | 2016-12-12 |
US Patents and Regulatory Information for VICTOZA
VICTOZA is protected by four US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of VICTOZA is ⤷ Try a Trial.
This potential generic entry date is based on patent ⤷ Try a Trial.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting VICTOZA
Needle mounting system and a method for mounting a needle assembly
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Propylene glycol-containing peptide formulations which are optimal for production and for use in injection devices
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Injection button
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Liraglutide in cardiovascular conditions
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Novo Nordisk Inc | VICTOZA | liraglutide recombinant | SOLUTION;SUBCUTANEOUS | 022341-001 | Jan 25, 2010 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Novo Nordisk Inc | VICTOZA | liraglutide recombinant | SOLUTION;SUBCUTANEOUS | 022341-001 | Jan 25, 2010 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Novo Nordisk Inc | VICTOZA | liraglutide recombinant | SOLUTION;SUBCUTANEOUS | 022341-001 | Jan 25, 2010 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Novo Nordisk Inc | VICTOZA | liraglutide recombinant | SOLUTION;SUBCUTANEOUS | 022341-001 | Jan 25, 2010 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for VICTOZA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Novo Nordisk Inc | VICTOZA | liraglutide recombinant | SOLUTION;SUBCUTANEOUS | 022341-001 | Jan 25, 2010 | ⤷ Try a Trial | ⤷ Try a Trial |
| Novo Nordisk Inc | VICTOZA | liraglutide recombinant | SOLUTION;SUBCUTANEOUS | 022341-001 | Jan 25, 2010 | ⤷ Try a Trial | ⤷ Try a Trial |
| Novo Nordisk Inc | VICTOZA | liraglutide recombinant | SOLUTION;SUBCUTANEOUS | 022341-001 | Jan 25, 2010 | ⤷ Try a Trial | ⤷ Try a Trial |
| Novo Nordisk Inc | VICTOZA | liraglutide recombinant | SOLUTION;SUBCUTANEOUS | 022341-001 | Jan 25, 2010 | ⤷ Try a Trial | ⤷ Try a Trial |
| Novo Nordisk Inc | VICTOZA | liraglutide recombinant | SOLUTION;SUBCUTANEOUS | 022341-001 | Jan 25, 2010 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for VICTOZA
See the table below for patents covering VICTOZA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| China | 1951965 | ⤷ Try a Trial | |
| Slovenia | 3300721 | ⤷ Try a Trial | |
| China | 1882356 | Propylene glycol-containing peptide formulations which are optimal for production and for use in injection devices | ⤷ Try a Trial |
| Australia | 2008213084 | Injection button | ⤷ Try a Trial |
| Japan | 2002508162 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for VICTOZA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2209800 | 14C0085 | France | ⤷ Try a Trial | PRODUCT NAME: LIRAGLUTIDE ET INSULINE DEGLUDEC; REGISTRATION NO/DATE: EU/1/14/947 20140918 |
| 2209800 | C300698 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: INSULINE DEGLUDEC EN LIRAGLUTIDE; REGISTRATION NO/DATE: EU/1/14/94765041 2014120918 |
| 0944648 | 09C0054 | France | ⤷ Try a Trial | PRODUCT NAME: LIRAGLUTIDE; REGISTRATION NO/DATE IN FRANCE: EU/1/09/529/001 DU 20090630; REGISTRATION NO/DATE AT EEC: EU/1/09/529/001 DU 20090630 |
| 2597103 | 2017/015 | Ireland | ⤷ Try a Trial | PRODUCT NAME: COMBINATION OF INSULIN DEGLUDEC AND LIRAGLUTIDE; NAT REGISTRATION NO/DATE: EU/1/14/947 20140918; FIRST REGISTRATION NO/DATE: 65041 20140912 |
| 0944648 | C00944648/01 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: LIRAGLUTID; REGISTRATION NO/DATE: SWISSMEDIC 59329 11.12.2009 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


