VIBERZI Drug Patent Profile
✉ Email this page to a colleague
When do Viberzi patents expire, and when can generic versions of Viberzi launch?
Viberzi is a drug marketed by Abbvie and is included in one NDA. There are twenty-one patents protecting this drug and one Paragraph IV challenge.
This drug has one hundred and fifty-three patent family members in forty countries.
The generic ingredient in VIBERZI is eluxadoline. There are two drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the eluxadoline profile page.
DrugPatentWatch® Generic Entry Outlook for Viberzi
Viberzi was eligible for patent challenges on May 27, 2019.
There have been nineteen patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
There are two tentative approvals for the generic drug (eluxadoline), which indicates the potential for near-term generic launch.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for VIBERZI?
- What are the global sales for VIBERZI?
- What is Average Wholesale Price for VIBERZI?
Summary for VIBERZI
| International Patents: | 153 |
| US Patents: | 21 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 36 |
| Clinical Trials: | 2 |
| Patent Applications: | 300 |
| Drug Prices: | Drug price information for VIBERZI |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for VIBERZI |
| What excipients (inactive ingredients) are in VIBERZI? | VIBERZI excipients list |
| DailyMed Link: | VIBERZI at DailyMed |
Recent Clinical Trials for VIBERZI
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Temple University | Phase 2/Phase 3 |
| University of North Carolina, Chapel Hill | Phase 2 |
| Allergan | Phase 2 |
Pharmacology for VIBERZI
| Drug Class | mu-Opioid Receptor Agonist |
| Mechanism of Action | Opioid mu-Receptor Agonists |
Paragraph IV (Patent) Challenges for VIBERZI
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| VIBERZI | Tablets | eluxadoline | 75 mg and 100 mg | 206940 | 6 | 2019-05-28 |
US Patents and Regulatory Information for VIBERZI
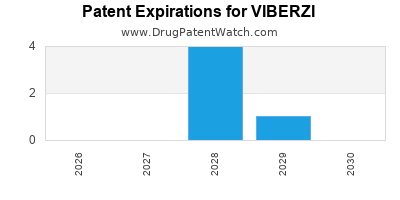
VIBERZI is protected by twenty-two US patents.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbvie | VIBERZI | eluxadoline | TABLET;ORAL | 206940-002 | May 27, 2015 | RX | Yes | Yes | 11,311,516 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Abbvie | VIBERZI | eluxadoline | TABLET;ORAL | 206940-002 | May 27, 2015 | RX | Yes | Yes | 9,675,587 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Abbvie | VIBERZI | eluxadoline | TABLET;ORAL | 206940-002 | May 27, 2015 | RX | Yes | Yes | 11,484,527 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Abbvie | VIBERZI | eluxadoline | TABLET;ORAL | 206940-002 | May 27, 2015 | RX | Yes | Yes | 7,786,158 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Abbvie | VIBERZI | eluxadoline | TABLET;ORAL | 206940-002 | May 27, 2015 | RX | Yes | Yes | 9,115,091 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Abbvie | VIBERZI | eluxadoline | TABLET;ORAL | 206940-001 | May 27, 2015 | RX | Yes | No | 9,115,091 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for VIBERZI
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Abbvie | VIBERZI | eluxadoline | TABLET;ORAL | 206940-001 | May 27, 2015 | 9,700,542 | ⤷ Get Started Free |
| Abbvie | VIBERZI | eluxadoline | TABLET;ORAL | 206940-002 | May 27, 2015 | 7,786,158 | ⤷ Get Started Free |
| Abbvie | VIBERZI | eluxadoline | TABLET;ORAL | 206940-002 | May 27, 2015 | 8,344,011 | ⤷ Get Started Free |
| Abbvie | VIBERZI | eluxadoline | TABLET;ORAL | 206940-001 | May 27, 2015 | 10,213,415 | ⤷ Get Started Free |
| Abbvie | VIBERZI | eluxadoline | TABLET;ORAL | 206940-002 | May 27, 2015 | 9,205,076 | ⤷ Get Started Free |
| Abbvie | VIBERZI | eluxadoline | TABLET;ORAL | 206940-002 | May 27, 2015 | 8,609,709 | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for VIBERZI
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Allergan Pharmaceuticals International Limited | Truberzi | eluxadoline | EMEA/H/C/004098Truberzi is indicated in adults for the treatment of irritable bowel syndrome with diarrhoea (IBS D). | Withdrawn | no | no | no | 2016-09-19 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for VIBERZI
When does loss-of-exclusivity occur for VIBERZI?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 14241076
Patent: Opioid receptor modulator dosage formulations
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2015022753
Patent: formulações de dosagens de moduladores de receptor opióide
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 06472
Patent: FORMULATIONS PHARMACEUTIQUES DE MODULATEUR DES RECEPTEURS AUX OPIOIDES (OPIOID RECEPTOR MODULATOR DOSAGE FORMULATIONS)
Estimated Expiration: ⤷ Get Started Free
China
Patent: 5228629
Patent: Opioid receptor modulator dosage formulations
Estimated Expiration: ⤷ Get Started Free
Patent: 0917159
Patent: 阿片样物质受体调节剂剂型 (Opioid receptor modulator dosage formulations)
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 20892
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 68351
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 1559
Patent: ДОЗИРОВАННЫЕ ПРЕПАРАТЫ В КАЧЕСТВЕ МОДУЛЯТОРОВ ОПИОИДНОГО РЕЦЕПТОРА (OPIOID RECEPTOR MODULATOR DOSAGE FORMULATIONS)
Estimated Expiration: ⤷ Get Started Free
Patent: 1591768
Patent: ДОЗИРОВАННЫЕ ПРЕПАРАТЫ В КАЧЕСТВЕ МОДУЛЯТОРОВ ОПИОИДНОГО РЕЦЕПТОРА
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 68351
Patent: FORMULATIONS PHARMACEUTIQUES DE MODULATEUR DES RÉCEPTEURS AUX OPIOÏDES (OPIOID RECEPTOR MODULATOR DOSAGE FORMULATIONS)
Estimated Expiration: ⤷ Get Started Free
Patent: 56321
Patent: FORMULATIONS DE DOSAGE DE MODULATEUR DU RÉCEPTEUR OPIOÏDE (OPIOID RECEPTOR MODULATOR DOSAGE FORMULATIONS)
Estimated Expiration: ⤷ Get Started Free
Patent: 65131
Patent: FORMULATIONS DE DOSAGE DE MODULATEUR DU RÉCEPTEUR OPIOÏDE (OPIOID RECEPTOR MODULATOR DOSAGE FORMULATIONS)
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 18514
Patent: 阿片受體調節劑劑型 (OPIOID RECEPTOR MODULATOR DOSAGE FORMULATIONS)
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 42748
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 1561
Patent: פורמולציה למינון מאפנן קולטן אופיאויד (Opioid receptor modulator dosage formulations)
Estimated Expiration: ⤷ Get Started Free
Patent: 8718
Patent: פורמולציה למינון מאפנן קולטן אופיאויד (Opioid receptor modulator dosage formulations)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 49225
Estimated Expiration: ⤷ Get Started Free
Patent: 66975
Estimated Expiration: ⤷ Get Started Free
Patent: 16516694
Patent: オピオイド受容体モジュレーターの投与製剤
Estimated Expiration: ⤷ Get Started Free
Patent: 19014744
Patent: オピオイド受容体モジュレーターの投与製剤 (DOSAGE FORMULATIONS OF OPIOID RECEPTOR MODULATOR)
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 68351
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 68351
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 68351
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 2435440
Estimated Expiration: ⤷ Get Started Free
Patent: 150140681
Patent: 오피오이드 수용체 조절인자 투여 제제 (OPIOID RECEPTOR MODULATOR DOSAGE FORMULATIONS)
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 93374
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 48071
Estimated Expiration: ⤷ Get Started Free
Patent: 1444590
Patent: Opioid receptor modulator dosage formulations
Estimated Expiration: ⤷ Get Started Free
Turkey
Patent: 1815953
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering VIBERZI around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| China | 105228629 | ⤷ Get Started Free | |
| Lithuania | 2653465 | ⤷ Get Started Free | |
| Taiwan | 201444590 | Opioid receptor modulator dosage formulations | ⤷ Get Started Free |
| Taiwan | I648071 | ⤷ Get Started Free | |
| Denmark | 2298744 | ⤷ Get Started Free | |
| China | 111620823 | 一种化合物的新型晶体及其制备方法 (Novel crystal of compound and method for preparing same) | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for VIBERZI
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1725537 | 1790007-7 | Sweden | ⤷ Get Started Free | PRODUCT NAME: ELUXADOLINE OR A PHARMACEUTICALLY ACCEPTABLE ENANTIOMER, DIASTEREOMER, RACEMATE OR SALT THEREOF; REG. NO/DATE: EU/1/16/1126 20160921 |
| 1725537 | 132017000028006 | Italy | ⤷ Get Started Free | PRODUCT NAME: ELUXADOLINE O UN ENANTIOMERO, DIASTEREOMERO, RACEMATO FARMACEUTICAMENTE ACCETTABILE O SALI DELLO STESSO(TRUBERZI); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/16/1126, 20160921 |
| 1725537 | 300865 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: ELUXADOLINE OF EEN FARMACEUTISCH AANVAARDBAAR ENANTIOMEER,DIASTEREOMEER, RACEMAAT OF ZOUT DAARVAN; REGISTRATION NO/DATE: EU/1/16/1126 20160921 |
| 2176234 | C02176234/01 | Switzerland | ⤷ Get Started Free | PRODUCT NAME: ELUXADOLIN; REGISTRATION NO/DATE: SWISSMEDIC-ZULASSUNG 66549 07.02.2018 |
| 1725537 | 309 6-2017 | Slovakia | ⤷ Get Started Free | PRODUCT NAME: ELUXADOLIN VO VSETKYCH FORMACH CHRANENYCH ZAKLADNYM PATENTOM; REGISTRATION NO/DATE: EU/1/16/1126 20160921 |
| 1725537 | PA2017005 | Lithuania | ⤷ Get Started Free | PRODUCT NAME: ELUKSADOLINAS ARBA JO FARMACINIU POZIURIU PRIIMTINAS ENANTIOMERAS, DIASTEREOMERAS, RACEMATAS ARBA DRUSKA; REGISTRATION NO/DATE: EU/1/16/1126 20160919 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for Viberzi (Eluxadoline)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
