TRULANCE Drug Patent Profile
✉ Email this page to a colleague
When do Trulance patents expire, and what generic alternatives are available?
Trulance is a drug marketed by Salix and is included in one NDA. There are ten patents protecting this drug and one Paragraph IV challenge.
This drug has seventy-five patent family members in twenty countries.
The generic ingredient in TRULANCE is plecanatide. One supplier is listed for this compound. Additional details are available on the plecanatide profile page.
DrugPatentWatch® Generic Entry Outlook for Trulance
Trulance was eligible for patent challenges on January 19, 2021.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be June 5, 2034. This may change due to patent challenges or generic licensing.
There have been eleven patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for TRULANCE?
- What are the global sales for TRULANCE?
- What is Average Wholesale Price for TRULANCE?
Summary for TRULANCE
| International Patents: | 75 |
| US Patents: | 10 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 15 |
| Clinical Trials: | 5 |
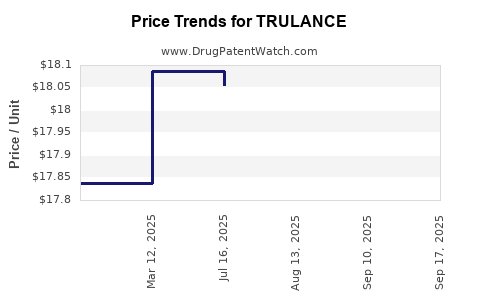
| Drug Prices: | Drug price information for TRULANCE |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for TRULANCE |
| What excipients (inactive ingredients) are in TRULANCE? | TRULANCE excipients list |
| DailyMed Link: | TRULANCE at DailyMed |
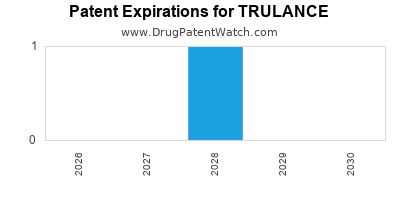
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for TRULANCE
Generic Entry Date for TRULANCE*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for TRULANCE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Synergy Pharmaceuticals Inc. | Phase 3 |
| Bausch Health Americas, Inc. | Phase 3 |
| Synergy Pharmaceuticals Inc. | Phase 2 |
Pharmacology for TRULANCE
| Drug Class | Guanylate Cyclase-C Agonist |
| Mechanism of Action | Guanylate Cyclase Activators |
Paragraph IV (Patent) Challenges for TRULANCE
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| TRULANCE | Tablets | plecanatide | 3 mg | 208745 | 2 | 2021-01-19 |
US Patents and Regulatory Information for TRULANCE
TRULANCE is protected by twelve US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of TRULANCE is ⤷ Get Started Free.
This potential generic entry date is based on patent 10,011,637.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Salix | TRULANCE | plecanatide | TABLET;ORAL | 208745-001 | Jan 19, 2017 | RX | Yes | Yes | 9,919,024 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Salix | TRULANCE | plecanatide | TABLET;ORAL | 208745-001 | Jan 19, 2017 | RX | Yes | Yes | 9,925,231 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Salix | TRULANCE | plecanatide | TABLET;ORAL | 208745-001 | Jan 19, 2017 | RX | Yes | Yes | 11,319,346 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Salix | TRULANCE | plecanatide | TABLET;ORAL | 208745-001 | Jan 19, 2017 | RX | Yes | Yes | 9,616,097 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Salix | TRULANCE | plecanatide | TABLET;ORAL | 208745-001 | Jan 19, 2017 | RX | Yes | Yes | 9,610,321 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for TRULANCE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Salix | TRULANCE | plecanatide | TABLET;ORAL | 208745-001 | Jan 19, 2017 | 7,799,897 | ⤷ Get Started Free |
| Salix | TRULANCE | plecanatide | TABLET;ORAL | 208745-001 | Jan 19, 2017 | 8,637,451 | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for TRULANCE
When does loss-of-exclusivity occur for TRULANCE?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 14274812
Estimated Expiration: ⤷ Get Started Free
Patent: 18226473
Estimated Expiration: ⤷ Get Started Free
Patent: 20205349
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2015030326
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 13737
Estimated Expiration: ⤷ Get Started Free
China
Patent: 5764916
Patent: 鸟苷酸环化酶C的超纯激动剂、制备和使用所述激动剂的方法 (Ultra-pure agonists of guanylate cyclase C, and method of making and using same)
Estimated Expiration: ⤷ Get Started Free
Patent: 3388007
Patent: 鸟苷酸环化酶C的超纯激动剂、制备和使用所述激动剂的方法 (Ultra-pure agonists of guanylate cyclase C, and methods of making and using same)
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0240805
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 1592263
Patent: УЛЬТРАЧИСТЫЕ АГОНИСТЫ ГУАНИЛАТЦИКЛАЗЫ C, СПОСОБ ИХ ПОЛУЧЕНИЯ И ИСПОЛЬЗОВАНИЯ
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 04138
Patent: AGONISTES ULTRA-PURS DE GUANYLATE CYCLASE C, LEUR PROCÉDÉ DE PRODUCTION ET D'UTILISATION (ULTRA-PURE AGONISTS OF GUANYLATE CYCLASE C, METHOD OF MAKING AND USING SAME)
Estimated Expiration: ⤷ Get Started Free
Patent: 24697
Patent: AGONISTES ULTRA-PURS DE GUANYLATE CYCLASE C, LEUR PROCÉDÉ DE FABRICATION ET D'UTILISATION (ULTRA-PURE AGONISTS OF GUANYLATE CYCLASE C, METHOD OF MAKING AND USING SAME)
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 21959
Patent: 鳥苷酸環化酶 的超純激動劑、製備和使用所述激動劑的方法 (ULTRA-PURE AGONISTS OF GUANYLATE YCLASE , METHOD OF MAKING AND USING SAME)
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 2939
Patent: אגוניסטים של גואנילט ציקלז c בדרגת זקוק גבוה, שיטות לייצורם ושימושים בהם (Ultra-pure agonists of guanylate cyclase c, method of making and using same)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 06491
Estimated Expiration: ⤷ Get Started Free
Patent: 16522216
Patent: グアニル酸シクラーゼCの超高純度アゴニスト、その作成および使用方法
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 04138
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 04138
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 632
Patent: ULTRA-PREČIŠĆENI AGONISTI GUANILAT-CIKLAZE C, POSTUPAK NJIHOVE PRIPREME I UPOTREBE (ULTRA-PURE AGONISTS OF GUANYLATE CYCLASE C, METHOD OF MAKING AND USING SAME)
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 04138
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 2272746
Estimated Expiration: ⤷ Get Started Free
Patent: 160039577
Patent: 구아닐레이트 사이클라제 C의 초순수 작용제, 및 이의 제조 및 사용 방법 (ULTRA-PURE AGONISTS OF GUANYLATE CYCLASE C, METHOD OF MAKING AND USING SAME)
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 81866
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering TRULANCE around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| European Patent Office | 2621509 | PRÉPARATIONS D'AGONISTES DU GUANYLATE CYCLASE-C ET MÉTHODES D'UTILISATION (FORMULATIONS OF GUANYLATE CYCLASE C AGONISTS AND METHODS OF USE) | ⤷ Get Started Free |
| Germany | 60233040 | ⤷ Get Started Free | |
| Canada | 2905585 | AGONISTES RECEPTEURS DE GUANYLATE CYCLASE DESTINES AU TRAITEMENT OU A LA PREVENTION DU CANCER OU DE POLYPES (GUANYLATE CYCLASE RECEPTOR AGONISTS FOR THE TREATMENT AND/OR PREVENTION OF CANCER OR POLYPS) | ⤷ Get Started Free |
| Hong Kong | 1221959 | 鳥苷酸環化酶 的超純激動劑、製備和使用所述激動劑的方法 (ULTRA-PURE AGONISTS OF GUANYLATE YCLASE , METHOD OF MAKING AND USING SAME) | ⤷ Get Started Free |
| Australia | 2011302006 | Formulations of guanylate cyclase C agonists and methods of use | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Market Dynamics and Financial Trajectory for the Pharmaceutical Drug: TRULANCE
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
