TRIJARDY XR Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Trijardy Xr, and when can generic versions of Trijardy Xr launch?
Trijardy Xr is a drug marketed by Boehringer Ingelheim and is included in one NDA. There are fifteen patents protecting this drug and one Paragraph IV challenge.
This drug has four hundred and eighty-five patent family members in forty-eight countries.
The generic ingredient in TRIJARDY XR is empagliflozin; linagliptin; metformin hydrochloride. There are twenty-two drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the empagliflozin; linagliptin; metformin hydrochloride profile page.
DrugPatentWatch® Generic Entry Outlook for Trijardy Xr
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be May 21, 2030. This may change due to patent challenges or generic licensing.
There have been twenty-one patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
Summary for TRIJARDY XR
| International Patents: | 485 |
| US Patents: | 15 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for TRIJARDY XR |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for TRIJARDY XR |
| What excipients (inactive ingredients) are in TRIJARDY XR? | TRIJARDY XR excipients list |
| DailyMed Link: | TRIJARDY XR at DailyMed |
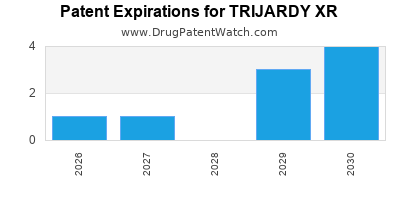
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for TRIJARDY XR
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Pharmacology for TRIJARDY XR
Anatomical Therapeutic Chemical (ATC) Classes for TRIJARDY XR
Paragraph IV (Patent) Challenges for TRIJARDY XR
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| TRIJARDY XR | Extended-release Tablets | empagliflozin; linagliptin; metformin hydrochloride | 5 mg/2.5 mg/1 g, 10 mg/5 mg/1 g, 12.5 mg/5 mg/1 g, 25 mg/5 mg/1 g | 212614 | 1 | 2020-05-26 |
US Patents and Regulatory Information for TRIJARDY XR
TRIJARDY XR is protected by seventeen US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of TRIJARDY XR is ⤷ Sign Up.
This potential generic entry date is based on patent ⤷ Sign Up.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting TRIJARDY XR
DPP-IV inhibitor combined with a further antidiabetic agent, tablets comprising such formulations, their use and process for their preparation
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: METHOD OF TREATING TYPE 2 DIABETES USING A PHARMACEUTICAL COMPOSITION COMPRISING LINAGLIPTIN, METFORMIN, EMPAGLIFLOZIN AND A BASIC AMINO ACID
Pharmaceutical composition, methods for treating and uses thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Pharmaceutical composition, methods for treating and uses thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: METHOD OF TREATING A TYPE 2 DIABETES MELLITUS PATIENT WITH INSUFFICIENT GLYCEMIC CONTROL DESPITE THERAPY WITH METFORMIN USING A PHARMACEUTICAL COMPOSITION COMPRISING EMPAGLIFLOZIN, LINAGLIPTIN AND METFORMIN
Pharmaceutical compositions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF A TREATMENT-NAIVE PATIENT WITH INADEQUATELY CONTROLLED TYPE 2 DIABETES USING A COMPOSITION COMPRISING AN EXTENDED RELEASE CORE COMPRISING METFORMIN AND AN OUTER COATING COMPRISING EMPAGLIFLOZIN AND LINAGLIPTIN
Pharmaceutical compositions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF A TYPE 2 DIABETES MELLITUS PATIENT WITH INSUFFICIENT GLYCEMIC CONTROL DESPITE METFORMIN THERAPY USING A COMPOSITION COMPRISING AN EXTENDED RELEASE CORE COMPRISING METFORMIN AND AN OUTER COATING COMPRISING EMPAGLIFLOZIN AND LINAGLIPTIN
Pharmaceutical composition, methods for treating and uses thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF A TYPE 2 DIABETES PATIENT WITH INSUFFICIENT GLYCEMIC CONTROL DESPITE METFORMIN THERAPY USING A COMPOSITION COMPRISING AN EXTENDED RELEASE CORE COMPRISING METFORMIN AND AN OUTER COATING COMPRISING EMPAGLIFLOZIN AND LINAGLIPTIN
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATING TYPE 2 DIABETES MELLITUS BY ASSESSING RENAL FUNCTION AND ORALLY ADMINISTERING EMPAGLIFLOZIN IN A DAILY AMOUNT OF 10 MG OR 25 MG IF THE EGFR IS >=30 ML/MIN/1.73 M2 AND
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATING TYPE 2 DIABETES MELLITUS BY ASSESSING RENAL FUNCTION AND ORALLY ADMINISTERING EMPAGLIFLOZIN IN A DAILY AMOUNT OF 10 MG OR 25 MG IF THE EGFR>=45 ML/MIN/1.73 M2 AND
8-[3-amino-piperidin-1-yl]-xanthines, the preparation thereof and their use as pharmaceutical compositions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Glucopyranosyl-substituted phenyl derivatives, medicaments containing such compounds, their use and process for their manufacture
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Crystalline form of 1-chloro-4-(.beta.-D-glucopyranos-1-yl)-2-[4-((S)-tetrahydrofuran-3-yloxy- )-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Pharmaceutical composition comprising a glucopyranosyl-substituted benzene derivate
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Process for the preparation of chiral 8-(3-aminopiperidin-1-yl)-xanthines
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
DPP-IV inhibitor combined with a further antidiabetic agent, tablets comprising such formulations, their use and process for their preparation
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
DPP-IV inhibitor combined with a further antidiabetic agent, tablets comprising such formulations, their use and process for their preparation
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Pharmaceutical composition, methods for treating and uses thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boehringer Ingelheim | TRIJARDY XR | empagliflozin; linagliptin; metformin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 212614-002 | Jan 27, 2020 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Boehringer Ingelheim | TRIJARDY XR | empagliflozin; linagliptin; metformin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 212614-003 | Jan 27, 2020 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Boehringer Ingelheim | TRIJARDY XR | empagliflozin; linagliptin; metformin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 212614-004 | Jan 27, 2020 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Boehringer Ingelheim | TRIJARDY XR | empagliflozin; linagliptin; metformin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 212614-003 | Jan 27, 2020 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Boehringer Ingelheim | TRIJARDY XR | empagliflozin; linagliptin; metformin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 212614-003 | Jan 27, 2020 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Boehringer Ingelheim | TRIJARDY XR | empagliflozin; linagliptin; metformin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 212614-004 | Jan 27, 2020 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for TRIJARDY XR
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Boehringer Ingelheim | TRIJARDY XR | empagliflozin; linagliptin; metformin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 212614-004 | Jan 27, 2020 | ⤷ Sign Up | ⤷ Sign Up |
| Boehringer Ingelheim | TRIJARDY XR | empagliflozin; linagliptin; metformin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 212614-003 | Jan 27, 2020 | ⤷ Sign Up | ⤷ Sign Up |
| Boehringer Ingelheim | TRIJARDY XR | empagliflozin; linagliptin; metformin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 212614-001 | Jan 27, 2020 | ⤷ Sign Up | ⤷ Sign Up |
| Boehringer Ingelheim | TRIJARDY XR | empagliflozin; linagliptin; metformin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 212614-001 | Jan 27, 2020 | ⤷ Sign Up | ⤷ Sign Up |
| Boehringer Ingelheim | TRIJARDY XR | empagliflozin; linagliptin; metformin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 212614-004 | Jan 27, 2020 | ⤷ Sign Up | ⤷ Sign Up |
| Boehringer Ingelheim | TRIJARDY XR | empagliflozin; linagliptin; metformin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 212614-002 | Jan 27, 2020 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for TRIJARDY XR
When does loss-of-exclusivity occur for TRIJARDY XR?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 1175
Estimated Expiration: ⤷ Sign Up
Australia
Patent: 09232043
Estimated Expiration: ⤷ Sign Up
Brazil
Patent: 0911273
Estimated Expiration: ⤷ Sign Up
Canada
Patent: 20450
Estimated Expiration: ⤷ Sign Up
Chile
Patent: 09000809
Estimated Expiration: ⤷ Sign Up
China
Patent: 1983073
Estimated Expiration: ⤷ Sign Up
Patent: 3083672
Estimated Expiration: ⤷ Sign Up
Patent: 6215190
Estimated Expiration: ⤷ Sign Up
Patent: 3648422
Estimated Expiration: ⤷ Sign Up
Colombia
Patent: 51277
Estimated Expiration: ⤷ Sign Up
Denmark
Patent: 85410
Estimated Expiration: ⤷ Sign Up
Ecuador
Patent: 10010489
Estimated Expiration: ⤷ Sign Up
Eurasian Patent Organization
Patent: 9395
Estimated Expiration: ⤷ Sign Up
Patent: 8435
Estimated Expiration: ⤷ Sign Up
Patent: 1001577
Estimated Expiration: ⤷ Sign Up
Patent: 1300121
Estimated Expiration: ⤷ Sign Up
European Patent Office
Patent: 85410
Estimated Expiration: ⤷ Sign Up
Patent: 53403
Estimated Expiration: ⤷ Sign Up
Patent: 44374
Estimated Expiration: ⤷ Sign Up
Hong Kong
Patent: 49485
Estimated Expiration: ⤷ Sign Up
Hungary
Patent: 41649
Estimated Expiration: ⤷ Sign Up
Japan
Patent: 88428
Estimated Expiration: ⤷ Sign Up
Patent: 22068
Estimated Expiration: ⤷ Sign Up
Patent: 11516456
Estimated Expiration: ⤷ Sign Up
Patent: 13237707
Estimated Expiration: ⤷ Sign Up
Mexico
Patent: 1232
Estimated Expiration: ⤷ Sign Up
Patent: 10010819
Estimated Expiration: ⤷ Sign Up
Morocco
Patent: 200
Estimated Expiration: ⤷ Sign Up
New Zealand
Patent: 7747
Estimated Expiration: ⤷ Sign Up
Patent: 9580
Estimated Expiration: ⤷ Sign Up
Peru
Patent: 091730
Estimated Expiration: ⤷ Sign Up
Patent: 140960
Estimated Expiration: ⤷ Sign Up
Poland
Patent: 85410
Estimated Expiration: ⤷ Sign Up
South Africa
Patent: 1005664
Estimated Expiration: ⤷ Sign Up
South Korea
Patent: 1611314
Estimated Expiration: ⤷ Sign Up
Patent: 1775942
Estimated Expiration: ⤷ Sign Up
Patent: 110005690
Estimated Expiration: ⤷ Sign Up
Patent: 160042174
Estimated Expiration: ⤷ Sign Up
Patent: 170056021
Estimated Expiration: ⤷ Sign Up
Spain
Patent: 96124
Estimated Expiration: ⤷ Sign Up
Taiwan
Patent: 27816
Estimated Expiration: ⤷ Sign Up
Patent: 0946534
Estimated Expiration: ⤷ Sign Up
Patent: 1509941
Estimated Expiration: ⤷ Sign Up
Tunisia
Patent: 10000431
Estimated Expiration: ⤷ Sign Up
Turkey
Patent: 1818886
Estimated Expiration: ⤷ Sign Up
Ukraine
Patent: 4136
Estimated Expiration: ⤷ Sign Up
Uruguay
Patent: 747
Estimated Expiration: ⤷ Sign Up
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering TRIJARDY XR around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| China | 110251519 | DPP IV抑制剂的用途 (USES OF DPP IV INHIBITORS) | ⤷ Sign Up |
| Slovenia | 2187879 | ⤷ Sign Up | |
| Germany | 102004054054 | Verfahren zur Herstellung chiraler 8-(3-Amino-piperidin-1-yl)-xanthine | ⤷ Sign Up |
| Denmark | 2187879 | ⤷ Sign Up | |
| Israel | 166964 | 8-[3-AMINO-PIPERIDIN-1-YL]-XANTHINES, THE PRODUCTION THEREOF AND THE USE OF THE SAME AS MEDICAMENTS | ⤷ Sign Up |
| Mexico | 2013010187 | COMPOSICIONES FARMACEUTICAS QUE COMPRENDEN MERFORMINA Y UN INHIBIDOR DE DPP-4 O UN INHIBIDOR DE SGLT-2. (PHARMACEUTICAL COMPOSITIONS COMPRISING METFORMIN AND A DPP-4 INHIBITOR OR A SGLT-2 INHIBITOR.) | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for TRIJARDY XR
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2187879 | PA2017014 | Lithuania | ⤷ Sign Up | PRODUCT NAME: EMPAGLIFLOZINO IR LINAGLIPTINO DERINYS ARBA JO FARMACINIU POZIURIU PRIIMTINA DRUSKA; REGISTRATION NO/DATE: EU/1/16/1146/001 - EU/1/16/1146/018 20161111 |
| 1730131 | C01730131/02 | Switzerland | ⤷ Sign Up | PRODUCT NAME: EMPAGLIFLOZIN UND METFORMINHYDROCHLORID; REGISTRATION NO/DATE: SWISSMEDIC 65570 12.11.2015 |
| 2187879 | 1790019-2 | Sweden | ⤷ Sign Up | PRODUCT NAME: COMBINATION OF EMPAGLIFLOZIN AND LINAGLIPTIN OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF; REG. NO/DATE: EU/1/16/1146 20161115 |
| 1532149 | 118 5013-2011 | Slovakia | ⤷ Sign Up | PRODUCT NAME: LINAGLIPTIN; REGISTRATION NO/DATE: EU/1/11/707/001 - EU/1/11/707/011 20110824 |
| 1532149 | 92128 | Luxembourg | ⤷ Sign Up | PRODUCT NAME: 8-(3-AMINOPIPERIDIN-1-YL)-7-BUT-2-INYL-3-METHYL-1-(4-METHYLCHINAZOLIN-2-YLMETHYL)-3, 7-DIHYDROPURIN-2, 6-DION, LES ENANTIOMERES ET LEURS SELS, EN PARTICULIER LA LINAGLIPTINE COMBINEE AVEC DU CHLORHYDRATE DE METFORMINE. LINAGLIPTINE |
| 1730131 | 1490061-7 | Sweden | ⤷ Sign Up | PERIOD OF VALIDITY (FROM - UNTIL): 20250312 - 20290526 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


