TOVIAZ Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Toviaz, and what generic alternatives are available?
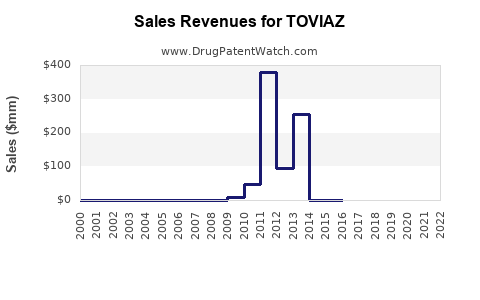
Toviaz is a drug marketed by Pfizer and is included in one NDA. There are three patents protecting this drug and one Paragraph IV challenge.
The generic ingredient in TOVIAZ is fesoterodine fumarate. There are fifteen drug master file entries for this compound. Fifteen suppliers are listed for this compound. Additional details are available on the fesoterodine fumarate profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Toviaz
A generic version of TOVIAZ was approved as fesoterodine fumarate by ALKEM LABS LTD on December 10th, 2015.
Summary for TOVIAZ
| US Patents: | 3 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 72 |
| Clinical Trials: | 25 |
| Patent Applications: | 142 |
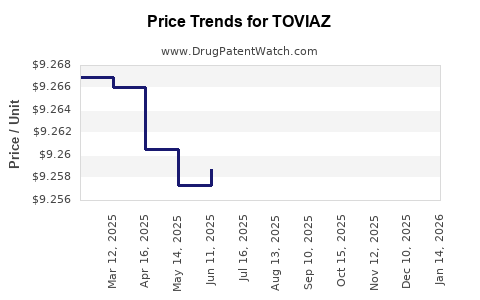
| Drug Prices: | Drug price information for TOVIAZ |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for TOVIAZ |
| What excipients (inactive ingredients) are in TOVIAZ? | TOVIAZ excipients list |
| DailyMed Link: | TOVIAZ at DailyMed |
Recent Clinical Trials for TOVIAZ
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Sir Mortimer B. Davis - Jewish General Hospital | Phase 4 |
| University of Alberta | Phase 2 |
| University of British Columbia | Phase 2 |
Anatomical Therapeutic Chemical (ATC) Classes for TOVIAZ
Paragraph IV (Patent) Challenges for TOVIAZ
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| TOVIAZ | Extended-release Tablets | fesoterodine fumarate | 4 mg and 8 mg | 022030 | 16 | 2012-10-31 |
US Patents and Regulatory Information for TOVIAZ
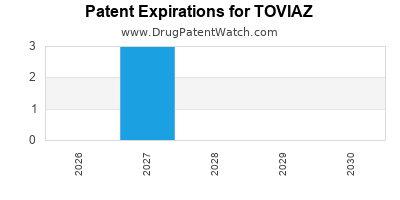
TOVIAZ is protected by three US patents and two FDA Regulatory Exclusivities.
Patents protecting TOVIAZ
Pharmaceutical compositions comprising fesoterodine
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Pharmaceutical compositions comprising fesoterodine
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Pharmaceutical compositions comprising fesoterodine
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
FDA Regulatory Exclusivity protecting TOVIAZ
TREATMENT OF NEUROGENIC DETRUSOR OVERACTIVITY (NDO) IN PEDIATRIC PATIENTS 6 YEARS OF AGE AND OLDER AND WEIGHING GREATER THAN 25 KG
Exclusivity Expiration: ⤷ Sign Up
PEDIATRIC EXCLUSIVITY
Exclusivity Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pfizer | TOVIAZ | fesoterodine fumarate | TABLET, EXTENDED RELEASE;ORAL | 022030-001 | Oct 31, 2008 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | ||
| Pfizer | TOVIAZ | fesoterodine fumarate | TABLET, EXTENDED RELEASE;ORAL | 022030-002 | Oct 31, 2008 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | ||
| Pfizer | TOVIAZ | fesoterodine fumarate | TABLET, EXTENDED RELEASE;ORAL | 022030-001 | Oct 31, 2008 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Pfizer | TOVIAZ | fesoterodine fumarate | TABLET, EXTENDED RELEASE;ORAL | 022030-002 | Oct 31, 2008 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | ||
| Pfizer | TOVIAZ | fesoterodine fumarate | TABLET, EXTENDED RELEASE;ORAL | 022030-001 | Oct 31, 2008 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | ||
| Pfizer | TOVIAZ | fesoterodine fumarate | TABLET, EXTENDED RELEASE;ORAL | 022030-001 | Oct 31, 2008 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | ||
| Pfizer | TOVIAZ | fesoterodine fumarate | TABLET, EXTENDED RELEASE;ORAL | 022030-002 | Oct 31, 2008 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for TOVIAZ
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Pfizer | TOVIAZ | fesoterodine fumarate | TABLET, EXTENDED RELEASE;ORAL | 022030-001 | Oct 31, 2008 | ⤷ Sign Up | ⤷ Sign Up |
| Pfizer | TOVIAZ | fesoterodine fumarate | TABLET, EXTENDED RELEASE;ORAL | 022030-002 | Oct 31, 2008 | ⤷ Sign Up | ⤷ Sign Up |
| Pfizer | TOVIAZ | fesoterodine fumarate | TABLET, EXTENDED RELEASE;ORAL | 022030-002 | Oct 31, 2008 | ⤷ Sign Up | ⤷ Sign Up |
| Pfizer | TOVIAZ | fesoterodine fumarate | TABLET, EXTENDED RELEASE;ORAL | 022030-002 | Oct 31, 2008 | ⤷ Sign Up | ⤷ Sign Up |
| Pfizer | TOVIAZ | fesoterodine fumarate | TABLET, EXTENDED RELEASE;ORAL | 022030-001 | Oct 31, 2008 | ⤷ Sign Up | ⤷ Sign Up |
| Pfizer | TOVIAZ | fesoterodine fumarate | TABLET, EXTENDED RELEASE;ORAL | 022030-002 | Oct 31, 2008 | ⤷ Sign Up | ⤷ Sign Up |
| Pfizer | TOVIAZ | fesoterodine fumarate | TABLET, EXTENDED RELEASE;ORAL | 022030-001 | Oct 31, 2008 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for TOVIAZ
See the table below for patents covering TOVIAZ around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Poland | 201422 | ⤷ Sign Up | |
| Poland | 356766 | ⤷ Sign Up | |
| Hungary | 230434 | 3,3-Difenil-propil-amin-származékok, eljárás elõállításukra, azokat tartalmazó gyógyászati készítmények és alkalmazásuk (Novel derivatives of 3,3-diphenylpropyl-amines, process for their production, their use and pharmaceutical compositions containing the same) | ⤷ Sign Up |
| Czech Republic | 302497 | Deriváty 3,3-difenylpropylaminu, zpusob jejich prípravy a použití (3,3-Diphenylpropylamine derivatives, process for their preparation and use) | ⤷ Sign Up |
| Norway | 20065380 | ⤷ Sign Up | |
| Portugal | 1230209 | ⤷ Sign Up | |
| South Africa | 200005728 | Novel derivatives of 3,3-diphenylpropylamines. | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for TOVIAZ
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1077912 | SPC037/2007 | Ireland | ⤷ Sign Up | SPC037/2007: 20080507, EXPIRES: 20220419 |
| 1230209 | PA2007008,C1230209 | Lithuania | ⤷ Sign Up | PRODUCT NAME: FESOTERODINUM; REGISTRATION NO/DATE: EU/1/07/386/001 - EU/1/07/386/010 20070420 |
| 1077912 | 308 | Finland | ⤷ Sign Up | |
| 1077912 | CA 2007 00046 | Denmark | ⤷ Sign Up | PRODUCT NAME: FESOTERODIN, FUMARAT |
| 1077912 | SZ 47/2007 | Austria | ⤷ Sign Up | PRODUCT NAME: FESOTERODINE UND IHRE SALZE MIT PHYSIOLOGISCH ANNEHMBAREN SAEUREN, EINSCHLIESSLICH FUMARSAEURE |
| 1481964 | C 2007 098 | Romania | ⤷ Sign Up | PRODUCT NAME: FUMARATACID DE FESOTERODINA IN FORMA CRISTALINA 2[(1R)-3-(DIIZOPROPILAMINO)-1-FENILPROPIL]-4-(HIDROXIMETIL)FENILIZOBUTIRIC INFORMA CRISTALINA - FUMARAT ACID DE FESOTERODINA IN FORMA CRISTALINA; NATIONAL AUTHORISATION NUMBER: RO EU/1/07/386/001, RO EU/1/07/386/002, RO EU/1/07/386/003, RO EU/1/07/386/004, RO EU/1/07/386/005, RO EU/1/07/386/006, RO EU/1/07/386/007, RO EU/1/07/386/008, RO EU/1/07/386/009, RO EU/1/07/386/010; DATE OF NATIONAL AUTHORISATION: 20070420; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/07/386/001, EU/1/07/386/002, EU/1/07/386/003, EU/1/07/386/004, EU/1/07/386/005, EU/1/07/386/006, EU/1/07/386/007, EU/1/07/386/0 [...] |
| 1077912 | C01077912/01 | Switzerland | ⤷ Sign Up | PRODUCT NAME: FESOTERODIN; REGISTRATION NUMBER/DATE: SWISSMEDIC 58743 18.12.2008 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


