Last updated: July 27, 2025
rket Dynamics and Financial Trajectory for TASIMELTEON: A Comprehensive Overview
Introduction
Tasimelteon, marketed under the brand name Hetlioz, is a novel oral melatonin receptor agonist primarily indicated for the treatment of non-24-hour sleep-wake disorder (Non-24) in totally blind individuals. Originally developed by Vanda Pharmaceuticals, tasimelteon represents a promising therapeutic agent in the sleep disorder landscape, with its unique mechanism targeting circadian rhythm regulation. This article delineates the current market dynamics influencing tasimelteon and scrutinizes its financial trajectory based on clinical, regulatory, and commercial developments.
Pharmacological Profile and Clinical Position
Tasimelteon acts as a selective agonist at melatonin receptors MT1 and MT2, which play critical roles in circadian rhythm entrainment. Its FDA approval in 2014 for Non-24 marked a milestone, granting it exclusivity in an area with no other targeted therapies. The drug’s efficacy hinges on realigning circadian rhythms in blind individuals lacking light perception, thus filling a crucial unmet medical need.
However, despite its targeted mechanism, the clinical adoption of tasimelteon faces hurdles. The rarity of non-24, affecting approximately 8,000 to 10,000 patients in the U.S., constrains its immediate market. Yet, the high unmet need affirms its value proposition, particularly within niche sleep disorders and circadian rhythm management.
Current Market Dynamics
1. Demographics and Patient Population:
Non-24 primarily affects totally blind individuals, representing a small but significant market segment. The limited prevalence constrains drug sales but establishes a high-value niche, especially given the lack of alternative treatments. Growth in diagnosed cases, driven by increased awareness and improved diagnostic protocols, could gradually expand the market.
2. Regulatory Environment:
The FDA granted tasimelteon Orphan Drug Designation, boosting its stature by providing benefits such as market exclusivity for seven years. Additionally, the potential for breakthrough therapy designation or additional indications, such as shift work disorder or delayed sleep phase syndrome, remains a strategic consideration.
3. Competition and Market Fragmentation:
The sleep disorder market is highly competitive with established treatments like melatonin supplements and behavioral therapies. However, these lack circadian rhythm resetting capabilities. Currently, no direct competitors to tasimelteon exist explicitly targeting non-24, although off-label melatonin and other chronobiological agents are used.
4. Clinical Expansion and Off-label Opportunities:
Efforts to demonstrate efficacy in other circadian-related conditions could bolster demand. Vanda’s ongoing or proposed trials to extend indications could significantly influence market penetration.
5. Market Penetration Challenges:
Limited awareness among physicians and patients, coupled with high treatment costs and reimbursement issues, impede widespread adoption. Moreover, the narrow indication confines revenue streams, emphasizing the importance of expanding approved uses.
Financial Trajectory and Revenue Outlook
1. Revenue Generation and Sales Trends:
Since its FDA approval, tasimelteon has seen steady, yet modest, revenue growth. Vanda Pharmaceuticals reported revenues of approximately $82 million in 2021, with a significant portion derived from Hetlioz sales. The growth trajectory remains sensitive to factors like new indications, expanded patient access, and market penetration efficiency.
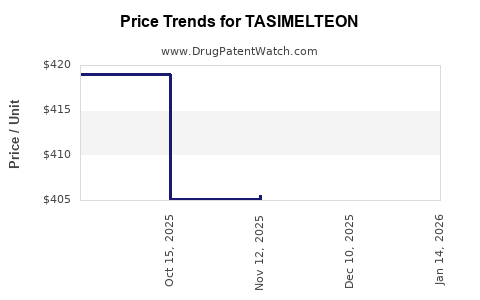
2. Pricing and Reimbursement:
Tasimelteon’s high cost, driven by its orphan status and specialized formulation, positions it as a premium therapy. Securing reimbursement from insurers is essential; however, coverage varies by region and payer policies. As access improves, revenue prospects could rise proportionally.
3. Patent and Market Exclusivity:
Vanda holds patents protecting tasimelteon until 2029, preserving competitive advantage. Patent expirations could open opportunities for generics, which would significantly impact revenue potential.
4. Strategic Partnerships and Licensing:
Potential licensing deals for broader indications and geographic expansion could accelerate sales. Moreover, collaborations with biosimilar manufacturers may influence long-term pricing strategies.
5. Market Expansion and Future Revenue Streams:
Identifying new indications, such as circadian rhythm disorders in shift workers or jet lag, could diversify revenue streams. Additionally, exploring pediatric applications and other sleep-related conditions may augment market size.
Emerging Trends and Future Outlook
- Pipeline Development: There is ongoing interest in developing next-generation melatonin receptor agents, which could either complement or challenge tasimelteon’s market position.
- Regulatory Pathways: Expedited review programs, including Orphan Drug and Fast Track designations, could hasten market access for new indications.
- Digital Health Integration: Incorporating pharmacogenomics and digital health tools may enhance treatment adherence and efficacy assessment, potentially boosting commercial success.
Challenges and Opportunities
Challenges:
- Limited patient population and narrow indication restrict revenue potential.
- Competition from over-the-counter melatonin supplements, despite their inferior pharmacological profile.
- Pricing and reimbursement barriers hinder accessibility.
- Patent expirations threaten future exclusivity.
Opportunities:
- Expanding to broader circadian rhythm disorder markets.
- Developing combination therapies for sleep disorders.
- Strategic collaborations to enhance global market reach.
- Investment into personalized medicine approaches to identify optimal responders.
Conclusion
Tasimelteon’s market dynamics are characterized by a high-value niche with limited but steady growth potential. Its future financial trajectory depends on successful indication expansion, regulatory support, effective commercialization, and strategic positioning against emerging competitors. Despite these challenges, its unique mechanism and FDA approval provide a solid foundation for continued relevance in the sleep disorder market, with room for growth as awareness and clinical applications evolve.
Key Takeaways
- Limited Market, High Value: Tasimelteon’s focus on non-24 positions it as a specialized therapy with significant unmet needs but constrained patient access.
- Regulatory Advantages: Orphan designation and patent protection underpin its market exclusivity, supporting sustained revenue.
- Growth Potential in Indication Expansion: Introducing tasimelteon into additional circadian disorders can diversify income streams.
- Pricing and Reimbursement Crucial: High costs necessitate proactive payer engagement to facilitate patient access.
- Patent Expiry Risks and Competition: The potential rise of generics post-2029 calls for strategic planning to maintain market share.
FAQs
1. What is the primary therapeutic use of tasimelteon?
Tasimelteon is approved for the treatment of non-24-hour sleep-wake disorder in totally blind individuals, aiding circadian rhythm entrainment.
2. Are there any other indications for tasimelteon under investigation?
Yes, clinical trials are exploring its efficacy for other circadian rhythm disorders such as shift work disorder and delayed sleep phase syndrome.
3. How does tasimelteon differentiate itself from over-the-counter melatonin supplements?
Tasimelteon selectively targets melatonin receptors MT1 and MT2 with pharmacokinetic properties designed for therapeutic efficacy, unlike generic melatonin, which varies in absorption and receptor affinity.
4. What are the main challenges to tasimelteon’s market growth?
Limited patient population, high treatment costs, reimbursement hurdles, and impending patent expirations pose significant obstacles.
5. What is the outlook for tasimelteon’s revenue in the coming years?
Revenue growth remains steady but moderate, contingent upon indication expansion, increased market awareness, and strategic collaborations, with potential risk from patent expirations around 2029.
Sources
[1] Vanda Pharmaceuticals Annual Reports, 2021.
[2] FDA Drug Approvals Database, 2014.
[3] MarketResearch.com, Sleep Disorder Therapeutics Market Analysis, 2022.
[4] ClinicalTrials.gov, Tasimelteon Trials and Indications Tracker, 2022.
[5] IQVIA Prescription Data, 2021.