TASIGNA Drug Patent Profile
✉ Email this page to a colleague
When do Tasigna patents expire, and when can generic versions of Tasigna launch?
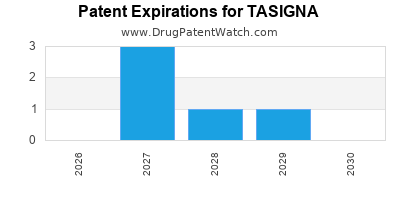
Tasigna is a drug marketed by Novartis and is included in one NDA. There are seven patents protecting this drug and two Paragraph IV challenges.
This drug has two hundred and ninety patent family members in fifty-two countries.
The generic ingredient in TASIGNA is nilotinib hydrochloride. There are eleven drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the nilotinib hydrochloride profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Tasigna
A generic version of TASIGNA was approved as nilotinib hydrochloride by APOTEX on January 5th, 2024.
Summary for TASIGNA
| International Patents: | 290 |
| US Patents: | 7 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 133 |
| Clinical Trials: | 67 |
| Patent Applications: | 4,662 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for TASIGNA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for TASIGNA |
| What excipients (inactive ingredients) are in TASIGNA? | TASIGNA excipients list |
| DailyMed Link: | TASIGNA at DailyMed |

Recent Clinical Trials for TASIGNA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Kartos Therapeutics, Inc. | Phase 1/Phase 2 |
| Baylor College of Medicine | Phase 2 |
| Ohio State University Comprehensive Cancer Center | Phase 1 |
Pharmacology for TASIGNA
Anatomical Therapeutic Chemical (ATC) Classes for TASIGNA
Paragraph IV (Patent) Challenges for TASIGNA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| TASIGNA | Capsules | nilotinib hydrochloride | 50 mg | 022068 | 1 | 2019-10-17 |
| TASIGNA | Capsules | nilotinib hydrochloride | 150 mg and 200 mg | 022068 | 1 | 2013-11-08 |
US Patents and Regulatory Information for TASIGNA
TASIGNA is protected by seven US patents and five FDA Regulatory Exclusivities.
Patents protecting TASIGNA
Inhibitors of tyrosine kinases
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Salts of 4-methyl-N-[3-(4-methyl-imidazol-1-yl)-5-trifluoromethyl-phenyl]-- 3-(4-pyridin-3-yl-pyrimidin-2-ylamino)-Benzamide
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Pharmaceutical compositions comprising nilotinib hydrochloride monohydrate
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Salts of 4-methyl N-[3-(4-methyl-imidazol-1-yl)-5-trifluoromethyl-phenyl]-3-(4-pyridin-3-yl- -pyrimidin-2-ylamino)-benzamide
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Crystalline forms of 4-methyl-N-[3-(4-methyl-imidazol-1-yl)-5-trifluoromethyl-phenyl]-3-(4-pyr- idin-3-yl-pyrimidin-2-ylamino)-benzamide
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Pharmaceutical compositions comprising nilotinib or its salt
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Method of treating proliferative disorders and other pathological conditions mediated by Bcr-Abl, c-Kit, DDR1, DDR2 or PDGF-R kinase activity
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
FDA Regulatory Exclusivity protecting TASIGNA
TREATMENT OF PEDIATRIC PATIENTS GREATER THAN OR EQUAL TO 1 YEAR OF AGE WITH NEWLY DIAGNOSED PHILADELPHIA CHROMOSOME POSITIVE CHRONIC MYELOID LEUKEMIA (PH+CML) IN CHRONIC PHASE
Exclusivity Expiration: ⤷ Sign Up
TREATMENT OF PEDIATRIC PATIENTS GREATER THAN OR EQUAL TO 1 YEAR OF AGE WITH CHRONIC PHASE PHILADELPHIA CHROMOSOME POSITIVE CHRONIC MYELOID LEUKEMIA WITH RESISTANCE OR INTOLERANCE TO PRIOR TYROSINE-KINASE INHIBITOR THERAPY
Exclusivity Expiration: ⤷ Sign Up
TREATMENT OF PEDIATRIC PATIENTS GREATER THAN OR EQUAL TO 1 YEAR OF AGE WITH ACCELERATED PHASE PHILADELPHIA CHROMOSOME POSITIVE CHRONIC MYELOID LEUKEMIA (PH+ CML) WITH RESISTANCE OR INTOLERANCE TO PRIOR TYROSINE-KINASE INHIBITOR (TKI) THERAPY
Exclusivity Expiration: ⤷ Sign Up
PEDIATRIC EXCLUSIVITY
Exclusivity Expiration: ⤷ Sign Up
PEDIATRIC EXCLUSIVITY
Exclusivity Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Novartis | TASIGNA | nilotinib hydrochloride | CAPSULE;ORAL | 022068-001 | Oct 29, 2007 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | ||
| Novartis | TASIGNA | nilotinib hydrochloride | CAPSULE;ORAL | 022068-001 | Oct 29, 2007 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | ||
| Novartis | TASIGNA | nilotinib hydrochloride | CAPSULE;ORAL | 022068-001 | Oct 29, 2007 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for TASIGNA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Novartis | TASIGNA | nilotinib hydrochloride | CAPSULE;ORAL | 022068-003 | Mar 22, 2018 | ⤷ Sign Up | ⤷ Sign Up |
| Novartis | TASIGNA | nilotinib hydrochloride | CAPSULE;ORAL | 022068-001 | Oct 29, 2007 | ⤷ Sign Up | ⤷ Sign Up |
| Novartis | TASIGNA | nilotinib hydrochloride | CAPSULE;ORAL | 022068-002 | Jun 17, 2010 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for TASIGNA
When does loss-of-exclusivity occur for TASIGNA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 9029
Estimated Expiration: ⤷ Sign Up
Australia
Patent: 10322102
Estimated Expiration: ⤷ Sign Up
Brazil
Patent: 2012011693
Estimated Expiration: ⤷ Sign Up
Canada
Patent: 79490
Estimated Expiration: ⤷ Sign Up
Chile
Patent: 12001270
Estimated Expiration: ⤷ Sign Up
China
Patent: 2612368
Estimated Expiration: ⤷ Sign Up
Colombia
Patent: 51690
Estimated Expiration: ⤷ Sign Up
Croatia
Patent: 0160472
Estimated Expiration: ⤷ Sign Up
Cyprus
Patent: 17519
Estimated Expiration: ⤷ Sign Up
Denmark
Patent: 01384
Estimated Expiration: ⤷ Sign Up
Ecuador
Patent: 12011903
Estimated Expiration: ⤷ Sign Up
European Patent Office
Patent: 01384
Estimated Expiration: ⤷ Sign Up
Finland
Patent: 01384
Estimated Expiration: ⤷ Sign Up
Guatemala
Patent: 1200150
Estimated Expiration: ⤷ Sign Up
Hong Kong
Patent: 69950
Estimated Expiration: ⤷ Sign Up
Hungary
Patent: 27307
Estimated Expiration: ⤷ Sign Up
Israel
Patent: 9727
Estimated Expiration: ⤷ Sign Up
Japan
Patent: 29615
Estimated Expiration: ⤷ Sign Up
Patent: 13511524
Estimated Expiration: ⤷ Sign Up
Patent: 15180636
Estimated Expiration: ⤷ Sign Up
Jordan
Patent: 34
Estimated Expiration: ⤷ Sign Up
Malaysia
Patent: 9956
Estimated Expiration: ⤷ Sign Up
Mexico
Patent: 12005694
Estimated Expiration: ⤷ Sign Up
Montenegro
Patent: 413
Estimated Expiration: ⤷ Sign Up
Morocco
Patent: 738
Estimated Expiration: ⤷ Sign Up
New Zealand
Patent: 9968
Estimated Expiration: ⤷ Sign Up
Peru
Patent: 121476
Estimated Expiration: ⤷ Sign Up
Poland
Patent: 01384
Estimated Expiration: ⤷ Sign Up
Russian Federation
Patent: 25835
Estimated Expiration: ⤷ Sign Up
Patent: 12124811
Estimated Expiration: ⤷ Sign Up
San Marino
Patent: 01600143
Estimated Expiration: ⤷ Sign Up
Serbia
Patent: 747
Estimated Expiration: ⤷ Sign Up
Singapore
Patent: 201501169V
Estimated Expiration: ⤷ Sign Up
Slovenia
Patent: 01384
Estimated Expiration: ⤷ Sign Up
South Africa
Patent: 1203328
Estimated Expiration: ⤷ Sign Up
South Korea
Patent: 1743315
Estimated Expiration: ⤷ Sign Up
Patent: 120102635
Estimated Expiration: ⤷ Sign Up
Spain
Patent: 72128
Estimated Expiration: ⤷ Sign Up
Taiwan
Patent: 98116
Estimated Expiration: ⤷ Sign Up
Patent: 1141481
Estimated Expiration: ⤷ Sign Up
Tunisia
Patent: 12000206
Estimated Expiration: ⤷ Sign Up
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering TASIGNA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Slovenia | 2284167 | ⤷ Sign Up | |
| Spain | 2475066 | ⤷ Sign Up | |
| Israel | 165977 | SUBSTITUTED PYRIMIDINYLAMINOBENZAMIDES , PROCESS FOR THE PREPARATION THEREOF AND PHARMACEUTICAL COMPOSITIONS COMPRISING THE SAME | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


