TASIGNA Drug Patent Profile
✉ Email this page to a colleague
When do Tasigna patents expire, and what generic alternatives are available?
Tasigna is a drug marketed by Novartis and is included in one NDA. There are six patents protecting this drug and two Paragraph IV challenges.
This drug has two hundred and thirty-one patent family members in fifty countries.
The generic ingredient in TASIGNA is nilotinib hydrochloride. There are eleven drug master file entries for this compound. Two suppliers are listed for this compound. Additional details are available on the nilotinib hydrochloride profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Tasigna
A generic version of TASIGNA was approved as nilotinib hydrochloride by APOTEX on January 5th, 2024.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for TASIGNA?
- What are the global sales for TASIGNA?
- What is Average Wholesale Price for TASIGNA?
Summary for TASIGNA
| International Patents: | 231 |
| US Patents: | 6 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 96 |
| Clinical Trials: | 68 |
| Patent Applications: | 305 |
| Drug Prices: | Drug price information for TASIGNA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for TASIGNA |
| What excipients (inactive ingredients) are in TASIGNA? | TASIGNA excipients list |
| DailyMed Link: | TASIGNA at DailyMed |
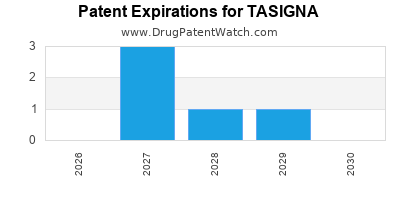

Paragraph IV (Patent) Challenges for TASIGNA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| TASIGNA | Capsules | nilotinib hydrochloride | 50 mg | 022068 | 1 | 2019-10-17 |
| TASIGNA | Capsules | nilotinib hydrochloride | 150 mg and 200 mg | 022068 | 1 | 2013-11-08 |
US Patents and Regulatory Information for TASIGNA
TASIGNA is protected by six US patents and five FDA Regulatory Exclusivities.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Novartis | TASIGNA | nilotinib hydrochloride | CAPSULE;ORAL | 022068-001 | Oct 29, 2007 | AB | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | ||
| Novartis | TASIGNA | nilotinib hydrochloride | CAPSULE;ORAL | 022068-001 | Oct 29, 2007 | AB | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Novartis | TASIGNA | nilotinib hydrochloride | CAPSULE;ORAL | 022068-001 | Oct 29, 2007 | AB | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | ||
| Novartis | TASIGNA | nilotinib hydrochloride | CAPSULE;ORAL | 022068-003 | Mar 22, 2018 | AB | RX | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | ||
| Novartis | TASIGNA | nilotinib hydrochloride | CAPSULE;ORAL | 022068-002 | Jun 17, 2010 | AB | RX | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for TASIGNA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Novartis | TASIGNA | nilotinib hydrochloride | CAPSULE;ORAL | 022068-003 | Mar 22, 2018 | ⤷ Get Started Free | ⤷ Get Started Free |
| Novartis | TASIGNA | nilotinib hydrochloride | CAPSULE;ORAL | 022068-002 | Jun 17, 2010 | ⤷ Get Started Free | ⤷ Get Started Free |
| Novartis | TASIGNA | nilotinib hydrochloride | CAPSULE;ORAL | 022068-001 | Oct 29, 2007 | ⤷ Get Started Free | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for TASIGNA
When does loss-of-exclusivity occur for TASIGNA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 9029
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 10322102
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2012011693
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 79490
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 12001270
Estimated Expiration: ⤷ Get Started Free
China
Patent: 2612368
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 51690
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0160472
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 17519
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 01384
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 12011903
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 01384
Estimated Expiration: ⤷ Get Started Free
Finland
Patent: 01384
Estimated Expiration: ⤷ Get Started Free
Guatemala
Patent: 1200150
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 69950
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 27307
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 9727
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 29615
Estimated Expiration: ⤷ Get Started Free
Patent: 13511524
Estimated Expiration: ⤷ Get Started Free
Patent: 15180636
Estimated Expiration: ⤷ Get Started Free
Jordan
Patent: 34
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 9956
Patent: METHOD OF TREATING PROLIFERATIVE DISORDERS AND OTHER PATHOLOGICAL CONDITIONS MEDIATED BY BCR-ABL, C-KIT, DDR1, DDR2 OR PDGF-R KINASE ACTIVITY
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 12005694
Patent: METODO PARA EL TRATAMIENTO DE TRANSTORNOS PROLIFERATIVOS Y OTRAS CONDICIONES PATOLOGICAS MEDIADAS POR LA ACTIVIDAD DE CINASA DE CBR-ABL, C-KIT, DDR1, DDR2 O PDGF-R. (METHOD OF TREATING PROLIFERATIVE DISORDERS AND OTHER PATHOLOGICAL CONDITIONS MEDIATED BY BCR-ABL, C-KIT, DDR1, DDR2 OR PDGF-R KINASE ACTIVITY.)
Estimated Expiration: ⤷ Get Started Free
Montenegro
Patent: 413
Patent: POSTUPAK LEČENJA PROLIFERATIVNIH OBOLJENJA I DRUGIH PATOLOŠKIH STANJA POSREDOVANIH AKTIVNOSCU BCR-ABL, C-KIT, DDR1, DDR2 ILI PDGF-R KINAZE (METHOD OF TREATING PROLIFERATIVE DISORDERS AND OTHER PATHOLOGICAL CONDITIONS MEDIATED BY BCR-ABL, C-KIT, DDR1, DDR2 OR PDGF-R KINASE ACTIVITY)
Estimated Expiration: ⤷ Get Started Free
Morocco
Patent: 738
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 9968
Patent: Method of treating proliferative disorders and other pathological conditions mediated by bcr-abl, c-kit, ddr1, ddr2 or pdgf-r kinase activity
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 121476
Patent: COMPOSICION PARA EL TRATAMIENTO DE TRASTORNOS PROLIFERATIVOS Y OTRAS CONDICIONES PATOLOGICAS MEDIADAS POR LA ACTIVIDAD DE CINASA DE BCR-ABL, C-KIT, DDR1, DDR2 O PDGF-R
Estimated Expiration: ⤷ Get Started Free
Philippines
Patent: 012500965
Patent: METHOD OF TREATING PROLIFERATIVE DISORDERS AND OTHER PATHOLOGICAL CONDITIONS MEDIATED BY BCR-ABL, C-KIT, DDR1, DDR2, OR PDGF-R KINASE ACTIVITY
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 01384
Estimated Expiration: ⤷ Get Started Free
Russian Federation
Patent: 25835
Patent: СПОСОБ ЛЕЧЕНИЯ ПРОЛИФЕРАТИВНЫХ НАРУШЕНИЙ И ДРУГИХ ПАТОЛОГИЧЕСКИХ СОСТОЯНИЙ, ОПОСРЕДОВАННЫХ АКТИВНОСТЬЮ КИНАЗ BCR-ABL, C-KIT, DDR1, DDR2 ИЛИ PDGF-R (METHOD FOR TREATING PROLIFERATIVE DISORDERS AND OTHER PATHOLOGICAL CONDITIONS MEDIATED BY ACTIVITY OF KINASE BCR-ABL, C-KIT, DDR1, DDR2 OR PDGF-R)
Estimated Expiration: ⤷ Get Started Free
Patent: 12124811
Patent: СПОСОБ ЛЕЧЕНИЯ ПРОЛИФЕРАТИВНЫХ НАРУШЕНИЙ И ДРУГИХ ПАТОЛОГИЧЕСКИХ СОСТОЯНИЙ, ОПОСРЕДОВАННЫХ АКТИВНОСТЬЮ КИНАЗ Bcr-Abl, c-Kit, DDR1, DDR2, ИЛИ PDGF-R
Estimated Expiration: ⤷ Get Started Free
San Marino
Patent: 01600143
Patent: METODO DI TRATTAMENTO DI DISTURBI PROLIFERATIVI E ALTRE CONDIZIONI PATOLOGICHE MEDIATE DALLA ATTIVITÀ CHINASICA DI BCR-ABL, C-KIT, DDR1, DDR2 O PDGF-R
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 747
Patent: POSTUPAK LEČENJA PROLIFERATIVNIH OBOLJENJA I DRUGIH PATOLOŠKIH STANJA POSREDOVANIM KINAZNOM AKTIVNOŠĆU BCR-ABL, C-KIT, DDR1, DDR2 ILI PDGF-R (METHOD OF TREATING PROLIFERATIVE DISORDERS AND OTHER PATHOLOGICAL CONDITIONS MEDIATED BY BCR-ABL, C-KIT, DDR1, DDR2 OR PDGF-R KINASE ACTIVITY)
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 201501169V
Patent: METHOD OF TREATING PROLIFERATIVE DISORDERS AND OTHER PATHOLOGICAL CONDITIONS MEDIATED BY BCR-ABL, C-KIT, DDR1, DDR2 OR PDGF-R KINASE ACTIVITY
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 01384
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1203328
Patent: METHOD OF TREATING PROLIFERATIVE DISORDERS AND OTHER PATHOLOGICAL CONDITIONS MEDIATED BY BCR-ABL, C-KIT, DDR1, DDR2 OR PDGF-R KINASE ACTIVITY
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1743315
Estimated Expiration: ⤷ Get Started Free
Patent: 120102635
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 72128
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 98116
Estimated Expiration: ⤷ Get Started Free
Patent: 1141481
Patent: Method of treating proliferative disorders and other pathological conditions mediated by Bcr-Abl, c-Kit, DDR1, DDR2 or PDGF-R kinase activity
Estimated Expiration: ⤷ Get Started Free
Tunisia
Patent: 12000206
Patent: METHOD OF TREATING PROLIFERATIVE DISORDERS AND OTHER PATHOLOGICAL CONDITIONS MEDIATED BY BCR-ABL, C-KIT, DDR1, DDR2 OR PDGF-R KINASE ACTIVITY
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering TASIGNA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| South Africa | 200710799 | Crystalline forms of 4-methyl-N-[3-(4-methyl-imidazol-1-yl)-5-trifluoromethylphenyl]-3-(4-pyridin-3-yl-pyrimidin-2-ylamino)-benzamide | ⤷ Get Started Free |
| New Zealand | 575317 | ⤷ Get Started Free | |
| South Korea | 20130077915 | CRYSTALLINE FORMS OF 4-METHYL-N-[3-(4-METHYL-IMIDAZOL-1-YL)-5-TRIFLUOROMETHYL-PHENYL]-3-(4-PYRIDIN-3-YL-PYRIMIDIN-2-YLAMINO)-BENZAMIDE | ⤷ Get Started Free |
| Spain | 2556625 | ⤷ Get Started Free | |
| Philippines | 12012500965 | METHOD OF TREATING PROLIFERATIVE DISORDERS AND OTHER PATHOLOGICAL CONDITIONS MEDIATED BY BCR-ABL, C-KIT, DDR1, DDR2, OR PDGF-R KINASE ACTIVITY | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Market Dynamics and Financial Trajectory for TASIGNA (Nilotinib)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
