SYMPROIC Drug Patent Profile
✉ Email this page to a colleague
When do Symproic patents expire, and when can generic versions of Symproic launch?
Symproic is a drug marketed by Bdsi and is included in one NDA. There are four patents protecting this drug.
This drug has seventy-four patent family members in twenty-seven countries.
The generic ingredient in SYMPROIC is naldemedine tosylate. One supplier is listed for this compound. Additional details are available on the naldemedine tosylate profile page.
DrugPatentWatch® Generic Entry Outlook for Symproic
Symproic was eligible for patent challenges on March 23, 2021.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be May 13, 2033. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
Summary for SYMPROIC
| International Patents: | 74 |
| US Patents: | 4 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 3 |
| Patent Applications: | 86 |
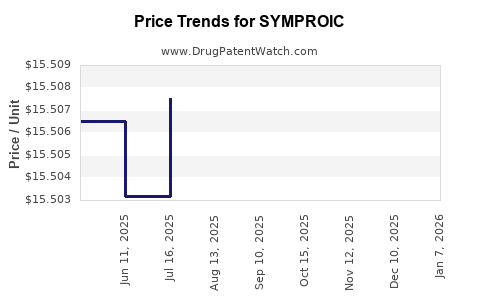
| Drug Prices: | Drug price information for SYMPROIC |
| What excipients (inactive ingredients) are in SYMPROIC? | SYMPROIC excipients list |
| DailyMed Link: | SYMPROIC at DailyMed |
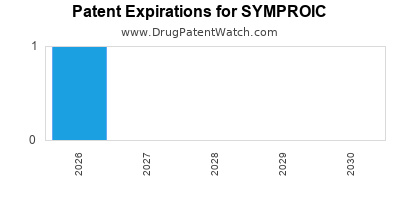
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for SYMPROIC
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Pharmacology for SYMPROIC
| Drug Class | Opioid Antagonist |
| Mechanism of Action | Opioid Antagonists |
Anatomical Therapeutic Chemical (ATC) Classes for SYMPROIC
US Patents and Regulatory Information for SYMPROIC
SYMPROIC is protected by four US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of SYMPROIC is ⤷ Sign Up.
This potential generic entry date is based on patent ⤷ Sign Up.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting SYMPROIC
Preparation containing 6,7-unsaturated-7-carbamoyl morphinan derivatives
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Crystal of 6,7-unsaturated-7-carbamoyl morphinan derivative and method for producing the same
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
6,7-unsaturated-7-carbamoyl substituted morphinan derivative
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
6,7-unsaturated-7-carbamoyl substituted morphinan derivative
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF OPIOID-INDUCED CONSTIPATION
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bdsi | SYMPROIC | naldemedine tosylate | TABLET;ORAL | 208854-001 | Mar 23, 2017 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| Bdsi | SYMPROIC | naldemedine tosylate | TABLET;ORAL | 208854-001 | Mar 23, 2017 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| Bdsi | SYMPROIC | naldemedine tosylate | TABLET;ORAL | 208854-001 | Mar 23, 2017 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| Bdsi | SYMPROIC | naldemedine tosylate | TABLET;ORAL | 208854-001 | Mar 23, 2017 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for SYMPROIC
When does loss-of-exclusivity occur for SYMPROIC?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Canada
Patent: 73961
Estimated Expiration: ⤷ Sign Up
Denmark
Patent: 51075
Estimated Expiration: ⤷ Sign Up
European Patent Office
Patent: 51075
Estimated Expiration: ⤷ Sign Up
Japan
Patent: 2013172297
Estimated Expiration: ⤷ Sign Up
Patent: 18219
Estimated Expiration: ⤷ Sign Up
Poland
Patent: 51075
Estimated Expiration: ⤷ Sign Up
Spain
Patent: 03149
Estimated Expiration: ⤷ Sign Up
Taiwan
Patent: 1350120
Estimated Expiration: ⤷ Sign Up
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering SYMPROIC around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Spain | 2746207 | ⤷ Sign Up | |
| Japan | 5191574 | ⤷ Sign Up | |
| World Intellectual Property Organization (WIPO) | 2013172297 | ⤷ Sign Up | |
| Philippines | 12015501226 | CRYSTALLINE 6,7-UNSATURATED-7-CARBAMOYL MORPHINANE DERIVATIVE, AND METHOD FOR PRODUCING THE SAME | ⤷ Sign Up |
| Japan | WO2013172297 | 6,7−不飽和−7−カルバモイルモルヒナン誘導体含有製剤 | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for SYMPROIC
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1889848 | C 2019 033 | Romania | ⤷ Sign Up | PRODUCT NAME: NALDEMEDINA SAU O SARE SAU SOLVAT ACCEPTABILE FARMACEUTIC; NATIONAL AUTHORISATION NUMBER: EU/1/18/1291; DATE OF NATIONAL AUTHORISATION: 20190218; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/18/1291; DATE OF FIRST AUTHORISATION IN EEA: 20190218 |
| 1889848 | C201930045 | Spain | ⤷ Sign Up | PRODUCT NAME: NADELMEDINA; NATIONAL AUTHORISATION NUMBER: EU/1/18/1291; DATE OF AUTHORISATION: 20190218; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/18/1291; DATE OF FIRST AUTHORISATION IN EEA: 20190218 |
| 1889848 | 38/2019 | Austria | ⤷ Sign Up | PRODUCT NAME: NALDEMEDINE ODER EIN PHARMAZEUTISCH ANNEHMBARES SALZ ODER SOLVAT DAVON, INSBESONDERE DAS TOSYLATSALZ; REGISTRATION NO/DATE: EU/1/18/1291 (MITTEILUNG) 20190220 |
| 1889848 | 2019/038 | Ireland | ⤷ Sign Up | PRODUCT NAME: NALDEMEDINE OR A PHARMACEUTICALLY ACCEPTABLE SALT OR SOLVATE THEREOF, IN PARTICULAR THE TOSYLATE SALT.; REGISTRATION NO/DATE: EU/1/18/1291 20190218 |
| 1889848 | CR 2019 00035 | Denmark | ⤷ Sign Up | PRODUCT NAME: NALDEMEDIN ELLER ET FARMACEUTISK ACCEPTABELT SALT ELLER SOLVAT DERAF, SAERLIGT TOSYLAT SALTET; REG. NO/DATE: EU/1/18/1291 20190220 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


