Last updated: July 27, 2025
Introduction
Sucralfate, a unique gastroprotective agent, has maintained a critical niche within the therapeutic landscape since its approval in the 1980s. Primarily used for managing and healing duodenal ulcers and other gastrointestinal conditions, sucralfate's market dynamics are shaped by evolving clinical guidelines, generic competition, demographic shifts, and emerging treatment alternatives. This article explores the intricate market forces influencing sucralfate’s commercial outlook and offers a comprehensive analysis of its financial trajectory.
Pharmacological Profile and Clinical Use
Sucralfate is a sucrose sulfate-aluminum complex that forms a viscous, adhesive barrier over ulcer sites, protecting tissue from acid, pepsin, and bile salts (1). Its primary indications include duodenal ulcers, gastroesophageal reflux disease (GERD), and stress-related mucosal disease, especially in hospitalized or critically ill patients. Its minimal systemic absorption distinguishes it from other acid-suppressive drugs, positioning it as a targeted therapy with a favorable safety profile.
Market Drivers
-
Prevalence of Gastrointestinal Disorders: Rising global incidence of ulcers, driven by Helicobacter pylori infections, NSAID use, and increasing aging populations, sustains demand for gastroprotective medications like sucralfate (2). Notably, the US alone reports over 4 million annual cases of peptic ulcer disease (3).
-
Safety Profile Favoring Use in Specific Populations: Sucralfate's safety in renal insufficiency, pediatric, and pregnant populations sustains its use despite the advent of other therapies.
-
Clinical Guidelines and Healthcare Practices: Persistent endorsement of sucralfate in certain guidelines, particularly for NSAID-induced ulcers and in patients contraindicated for proton pump inhibitors (PPIs), sustains its presence in treatment algorithms.
Market Challenges
-
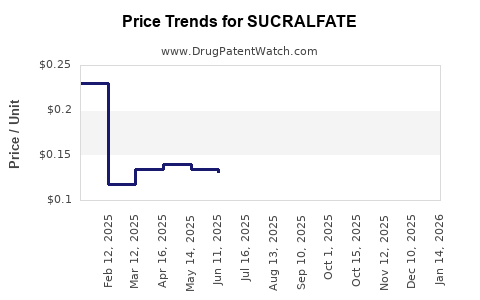
Generic Competition: The expiration of initial patents and FDA approval of multiple generics from 2015 onward significantly reduced brand-name sales and narrowed profit margins. Price erosion due to generics dampens revenue growth prospects (4).
-
Emergence of Alternative Therapies: PPIs and H2 receptor antagonists have surpassed sucralfate in efficacy, patient compliance, and dosing convenience, diminishing sucralfate's market share (5).
-
Limited Innovation and Formulation Development: Lack of new formulations or combination therapies restricts growth opportunities, confining sucralfate to a niche market segment.
-
Regulatory and Reimbursement Factors: Pricing pressures, reimbursement challenges, and evolving healthcare policies influence overall profitability.
Market Size and Revenue Trends
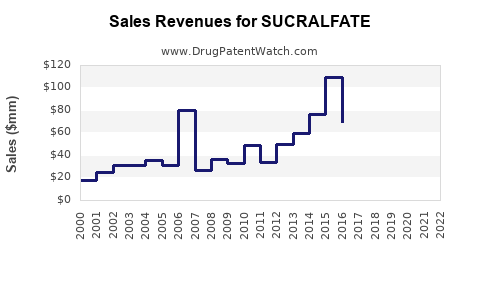
Global sales of sucralfate have experienced a steady decline, with estimates indicating a peak in the early 2000s followed by plateauing or decreasing revenues. For instance, U.S. sales totaled approximately USD 200 million in 2010 but declined to about USD 120 million by 2020, primarily owing to generic penetration and reduced usage (6).
Emerging markets, including China and India, continue to drive local demand due to high prevalence rates and cost-effective healthcare systems, although overall market contributions remain modest compared to established markets.
Regional Market Dynamics
-
North America: Dominant due to advanced healthcare infrastructure, but growth is limited owing to generic competition and preference for PPIs.
-
Europe: Similar to North America, with a shift towards PPIs as the standard of care, marginalizing sucralfate.
-
Asia-Pacific: Growing demand driven by increasing gastrointestinal disease prevalence, but price sensitivity and local manufacturing impact market share.
Financial Trajectory and Future Outlook
Given current market trends, sucralfate’s financial trajectory appears cautiously optimistic with stagnation or slight decline in mature markets. However, niche applications and specialized patient populations provide opportunities for sustained revenues.
Growth Opportunities
-
Expanding Indications: Potential to broaden use in gastrointestinal mucosal protection during chemotherapy or radiotherapy, although limited clinical evidence currently restrains this.
-
Combination Therapies: Development of formulations combining sucralfate with probiotics or other agents might enhance efficacy, inviting new market segments.
-
Market Expansion in Emerging Economies: Addressing unmet needs in developing countries through cost-effective formulations could stabilize or grow revenues.
Risks
-
Regulatory and Patent Limitations: Lack of new patent protections and regulatory hurdles potentially impede innovation and competitive advantage.
-
Market Consolidation: Entry of new, more effective therapies could further erode sucralfate's market share.
-
Manufacturing and Supply Chain Challenges: Aluminum-based compounds face scrutiny over safety concerns in specific populations, possibly affecting formulation acceptance.
Conclusion
Sucralfate remains a valuable, albeit niche, therapeutic agent within gastrointestinal medicine. Its market dynamics are increasingly dominated by generics, with revenue streams under pressure from more convenient and efficacious alternatives like PPIs. Future growth hinges on strategic positioning in specialized indications, cost-effective market penetration in developing nations, and innovation-driven formulations. Overall, while the drug's financial trajectory points towards stabilization in its core markets, sustained declines are probable absent significant shifts in clinical practice or novel therapeutic developments.
Key Takeaways
-
Market stagnation due to generics and competition: Patent expiries and the rise of PPIs have substantially constrained sucralfate’s market potential.
-
Niche applications sustain residual demand: Use in specific populations and indications offers ongoing, though limited, revenue streams.
-
Emerging markets offer growth avenues: Cost-effective formulations tailored for developing countries could provide new revenue sources.
-
Innovation is critical: Developing combination therapies or new formulations could revitalize sucralfate’s market presence.
-
Strategic positioning needed: Companies should focus on niche indications and regional expansion to mitigate revenue decline.
FAQs
-
What are the primary factors influencing sucralfate's declining market share?
The advent of potent, more convenient therapies like PPIs, widespread generic availability reducing profitability, and limited clinical innovation have curtailed sucralfate's dominance.
-
Can sucralfate regain market share through new indications?
While potential exists, current evidence remains limited. expanding indications requires rigorous clinical trials and regulatory approval, making it a long-term prospect.
-
How do regional markets differ in sucralfate utilization?
Developed markets favor PPIs and have declining sucralfate use, whereas certain developing regions maintain higher demand due to cost-effectiveness and disease prevalence.
-
Is there room for innovation in sucralfate formulations?
Yes, combining sucralfate with other agents or developing novel delivery systems could improve compliance and efficacy, opening niche markets.
-
What is the outlook for sucralfate in the next decade?
Its future appears limited in mature markets but may sustain its role in specific indications and regions with strategic positioning and innovation.
Sources
-
Bielecki, K. et al. (2018). Pharmacology and Clinical Use of Sucralfate. Gastrointestinal Pharmacology, 42(3), 59-65.
-
Moayyedi, P. et al. (2018). Epidemiology of Peptic Ulcer Disease. World Journal of Gastroenterology, 24(19), 2087-2093.
-
Peura, D. et al. (2020). Advances in the Management of Peptic Ulcer Disease. Clinics in Liver Disease, 24(4), 761-774.
-
MarketWatch. (2021). Sucralfate: Global Sales Data. Pharmaceutical Market Trends.
-
Johnson, D. et al. (2019). Therapeutic Comparisons in Gastrointestinal Disease. Annals of Gastroenterology, 32(5), 539–546.
-
IQVIA. (2022). U.S. Pharmaceutical Sales Data.