SUBOXONE Drug Patent Profile
✉ Email this page to a colleague
When do Suboxone patents expire, and when can generic versions of Suboxone launch?
Suboxone is a drug marketed by Indivior and is included in two NDAs. There are three patents protecting this drug and three Paragraph IV challenges.
This drug has thirty-two patent family members in twenty-four countries.
The generic ingredient in SUBOXONE is buprenorphine hydrochloride; naloxone hydrochloride. There are twenty-nine drug master file entries for this compound. Twenty-three suppliers are listed for this compound. Additional details are available on the buprenorphine hydrochloride; naloxone hydrochloride profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Suboxone
A generic version of SUBOXONE was approved as buprenorphine hydrochloride; naloxone hydrochloride by ACTAVIS ELIZABETH on February 22nd, 2013.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for SUBOXONE?
- What are the global sales for SUBOXONE?
- What is Average Wholesale Price for SUBOXONE?
Summary for SUBOXONE
| International Patents: | 32 |
| US Patents: | 3 |
| Applicants: | 1 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 30 |
| Clinical Trials: | 100 |
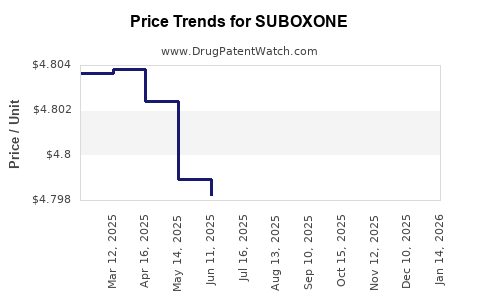
| Drug Prices: | Drug price information for SUBOXONE |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for SUBOXONE |
| What excipients (inactive ingredients) are in SUBOXONE? | SUBOXONE excipients list |
| DailyMed Link: | SUBOXONE at DailyMed |
Recent Clinical Trials for SUBOXONE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Indivior Inc. | PHASE2 |
| Erin Winstanley | PHASE4 |
| National Institute on Drug Abuse (NIDA) | PHASE4 |
Pharmacology for SUBOXONE
| Drug Class | Opioid Antagonist Partial Opioid Agonist |
| Mechanism of Action | Opioid Antagonists Partial Opioid Agonists |
Paragraph IV (Patent) Challenges for SUBOXONE
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| SUBOXONE | Sublingual Film | buprenorphine hydrochloride; naloxone hydrochloride | 12 mg/3 mg | 022410 | 1 | 2013-05-14 |
| SUBOXONE | Sublingual Film | buprenorphine hydrochloride; naloxone hydrochloride | 2 mg/0.5 mg* and 8 mg/2 mg | 022410 | 1 | 2012-10-15 |
| SUBOXONE | for Injection | buprenorphine hydrochloride; naloxone hydrochloride | 500 mg/vial | 020733 | 2 | 2009-01-26 |
US Patents and Regulatory Information for SUBOXONE
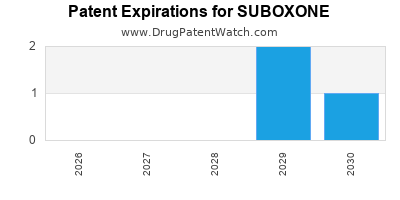
SUBOXONE is protected by three US patents.
Expired US Patents for SUBOXONE
International Patents for SUBOXONE
When does loss-of-exclusivity occur for SUBOXONE?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 8417
Patent: COMPOSICIONES DE PELICULA SUBLINGUAL Y BUCAL
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 10279440
Patent: Sublingual and buccal film compositions
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2012002817
Patent: composições sublinguais e bucais em filme
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 70180
Patent: COMPOSITIONS PELLICULAIRES SUBLINGUALES ET BUCCALES (SUBLINGUAL AND BUCCAL FILM COMPOSITIONS)
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 12000313
Patent: Composición de dosificación de película que comprende una matriz portadora, entre 2-16 mg de buprenorfina, entre 0,5-5 mg de naloxona y un tampón que proporciona un ph local entre 2-4; procedimiento de preparación; uso en el tratamiento de la dependencia de narcóticos en un usuario.
Estimated Expiration: ⤷ Get Started Free
China
Patent: 2548535
Patent: Sublingual and buccal film compositions
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 11219
Patent: COMPOSICIONES DE PELÍCULA SUBLINGUALES Y BUCALES
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0160368
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 61795
Estimated Expiration: ⤷ Get Started Free
Patent: 31445
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 61795
Patent: COMPOSITIONS PELLICULAIRES SUBLINGUALES ET BUCCALES (SUBLINGUAL AND BUCCAL FILM COMPOSITIONS)
Estimated Expiration: ⤷ Get Started Free
Patent: 31445
Patent: COMPOSITIONS PELLICULAIRES SUBLINGUALES ET BUCCALES (SUBLINGUAL AND BUCCAL FILM COMPOSITIONS)
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 71960
Patent: 舌下和口腔用薄膜組合物 (SUBLINGUAL AND BUCCAL FILM COMPOSITIONS)
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 7964
Patent: תכשירים בצורה שטוחה המכילים בופרנורפין ונלוקסון, פורמולציות בתצורה שטוחה המכילות אותם, ושימושן בהכנת תרופות לטיפול בתלות בחומרים נרקוטיים (Film dosage compositions comprising buprenorphine and naloxone, film formulations comprising the same and use thereof for the preparation of medicaments for treating narcotic dependence)
Estimated Expiration: ⤷ Get Started Free
Patent: 4974
Patent: תכשירים בצורה שטוחה המכילים בופרנורפין ונלוקסון, פורמולציות בתצורה שטוחה המכילות אותם, ושימושן בהכנת תרופות לטיפול בתלות בחומרים נרקוטיים (Film dosage compositions comprising buprenorphine and naloxone, film formulations comprising the same and use thereof for the preparation of medicaments for treating narcotic dependence)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 19827
Estimated Expiration: ⤷ Get Started Free
Patent: 13501717
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 6006
Patent: SUBLINGUAL AND BUCCAL FILM COMPOSITIONS
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 12001573
Patent: COMPOSICIONES DE PELÍCULA SUBLINGUALES Y BUCALES. (SUBLINGUAL AND BUCCAL FILM COMPOSITIONS.)
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 8537
Patent: Sublingual and buccal film compositions
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 121136
Patent: COMPOSICIONES DE PELICULA SUBLINGUALES Y BUCALES
Estimated Expiration: ⤷ Get Started Free
Russian Federation
Patent: 12108632
Patent: СУБЛИНГВАЛЬНЫЕ И БУККАЛЬНЫЕ ПЛЕНОЧНЫЕ КОМПОЗИЦИИ
Estimated Expiration: ⤷ Get Started Free
Patent: 18121855
Patent: СУБЛИНГВАЛЬНЫЕ И БУККАЛЬНЫЕ ПЛЕНОЧНЫЕ КОМПОЗИЦИИ
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 8265
Patent: SUBLINGUAL AND BUCCAL FILM COMPOSITIONS
Estimated Expiration: ⤷ Get Started Free
Patent: 201601214V
Patent: SUBLINGUAL AND BUCCAL FILM COMPOSITIONS
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1201647
Patent: SUBLINGUAL AND BUCCAL FILM COMPOSITIONS
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1699321
Estimated Expiration: ⤷ Get Started Free
Patent: 120059538
Patent: SUBLINGUAL AND BUCCAL FILM COMPOSITIONS
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 62634
Estimated Expiration: ⤷ Get Started Free
Patent: 57814
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering SUBOXONE around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| China | 1764443 | Thin film with non-self-aggregating uniform heterogeneity and drug delivery systems made therefrom | ⤷ Get Started Free |
| South Africa | 201201647 | SUBLINGUAL AND BUCCAL FILM COMPOSITIONS | ⤷ Get Started Free |
| Singapore | 178265 | ⤷ Get Started Free | |
| Colombia | 6511219 | COMPOSICIONES DE PELÍCULA SUBLINGUALES Y BUCALES | ⤷ Get Started Free |
| Canada | 2456234 | INSTRUMENTS OPTIQUES ACCORDABLES (TUNABLE OPTICAL INSTRUMENTS) | ⤷ Get Started Free |
| Norway | 20056060 | ⤷ Get Started Free | |
| Japan | 5748494 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
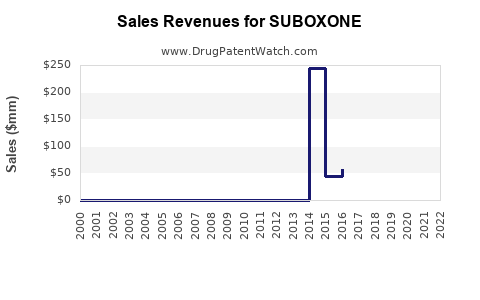
Market Dynamics and Financial Trajectory for SUBOXONE
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
