STRIVERDI RESPIMAT Drug Patent Profile
✉ Email this page to a colleague
When do Striverdi Respimat patents expire, and when can generic versions of Striverdi Respimat launch?
Striverdi Respimat is a drug marketed by Boehringer Ingelheim and is included in one NDA. There are nine patents protecting this drug.
This drug has one hundred and ninety-four patent family members in forty countries.
The generic ingredient in STRIVERDI RESPIMAT is olodaterol hydrochloride. One supplier is listed for this compound. Additional details are available on the olodaterol hydrochloride profile page.
DrugPatentWatch® Generic Entry Outlook for Striverdi Respimat
Striverdi Respimat was eligible for patent challenges on July 31, 2018.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be January 19, 2027. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
AI Research Assistant
Questions you can ask:
- What is the 5 year forecast for STRIVERDI RESPIMAT?
- What are the global sales for STRIVERDI RESPIMAT?
- What is Average Wholesale Price for STRIVERDI RESPIMAT?
Summary for STRIVERDI RESPIMAT
| International Patents: | 194 |
| US Patents: | 9 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 36 |
| Clinical Trials: | 2 |
| Patent Applications: | 39 |
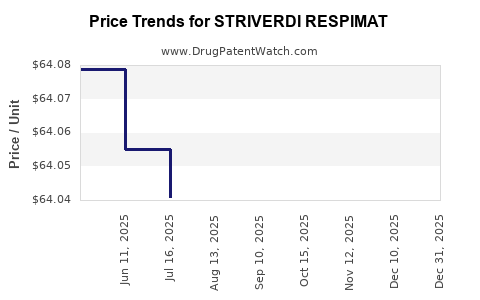
| Drug Prices: | Drug price information for STRIVERDI RESPIMAT |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for STRIVERDI RESPIMAT |
| What excipients (inactive ingredients) are in STRIVERDI RESPIMAT? | STRIVERDI RESPIMAT excipients list |
| DailyMed Link: | STRIVERDI RESPIMAT at DailyMed |
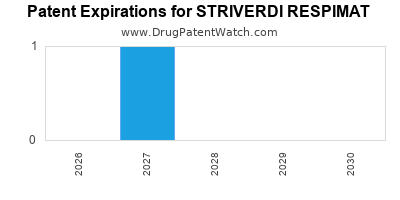
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for STRIVERDI RESPIMAT
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for STRIVERDI RESPIMAT
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Università degli Studi di Ferrara | Phase 4 |
| Boehringer Ingelheim |
Pharmacology for STRIVERDI RESPIMAT
| Drug Class | beta2-Adrenergic Agonist |
| Mechanism of Action | Adrenergic beta2-Agonists |
US Patents and Regulatory Information for STRIVERDI RESPIMAT
STRIVERDI RESPIMAT is protected by nine US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of STRIVERDI RESPIMAT is ⤷ Sign Up.
This potential generic entry date is based on patent ⤷ Sign Up.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting STRIVERDI RESPIMAT
Enantiomerically pure beta agonists, process for the manufacture thereof and use thereof as medicaments
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD), CHRONIC BRONCHITIS OR EMPHYSEMA
Piston-pumping system having o-ring seal properties
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Blocking device for a locking stressing mechanism having a spring-actuated output drive device
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD), CHRONIC BRONCHITIS OR EMPHYSEMA
Medicaments for the treatment of chronic obstructive pulmonary disease
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Device for clamping a fluidic component
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Microstructured high pressure nozzle with built-in filter function
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Enantiomerically pure beta agonists, process for the manufacture thereof and use thereof as medicaments
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD), CHRONIC BRONCHITIS OR EMPHYSEMA
Atomizer and method of atomizing fluid with a nozzle rinsing mechanism
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Device for clamping a fluidic component
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boehringer Ingelheim | STRIVERDI RESPIMAT | olodaterol hydrochloride | SPRAY, METERED;INHALATION | 203108-001 | Jul 31, 2014 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Boehringer Ingelheim | STRIVERDI RESPIMAT | olodaterol hydrochloride | SPRAY, METERED;INHALATION | 203108-001 | Jul 31, 2014 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Boehringer Ingelheim | STRIVERDI RESPIMAT | olodaterol hydrochloride | SPRAY, METERED;INHALATION | 203108-001 | Jul 31, 2014 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Boehringer Ingelheim | STRIVERDI RESPIMAT | olodaterol hydrochloride | SPRAY, METERED;INHALATION | 203108-001 | Jul 31, 2014 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Boehringer Ingelheim | STRIVERDI RESPIMAT | olodaterol hydrochloride | SPRAY, METERED;INHALATION | 203108-001 | Jul 31, 2014 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| Boehringer Ingelheim | STRIVERDI RESPIMAT | olodaterol hydrochloride | SPRAY, METERED;INHALATION | 203108-001 | Jul 31, 2014 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Boehringer Ingelheim | STRIVERDI RESPIMAT | olodaterol hydrochloride | SPRAY, METERED;INHALATION | 203108-001 | Jul 31, 2014 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for STRIVERDI RESPIMAT
International Patents for STRIVERDI RESPIMAT
See the table below for patents covering STRIVERDI RESPIMAT around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Japan | 4879439 | ⤷ Sign Up | |
| Uruguay | 26021 | CARTUCHO PARA UN LIQUIDO | ⤷ Sign Up |
| Brazil | 9611878 | ⤷ Sign Up | |
| Cyprus | 1110500 | ⤷ Sign Up | |
| South Africa | 9610182 | ⤷ Sign Up | |
| Japan | 2007517646 | ⤷ Sign Up | |
| Argentina | 003795 | DISPOSITIVO PARA SUJETAR UN COMPONENTE SOMETIDO A UN FLUIDO PRESURIZADO | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for STRIVERDI RESPIMAT
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1562603 | 14C0049 | France | ⤷ Sign Up | PRODUCT NAME: OLODATEROL, SES ISOMERES OPTIQUES INDIVIDUELS, MELANGES D'ENANTIOMERES INDIVIDUELS OU RACEMATE, SES SELS D'ADDITION D'ACIDE AVEC DES ACIDES PHARMACOLOGIQUEMENT ACCEPTABLES, AINSI QUE DES SOLVATES ET OU HYDRATES, EN PARTICULIER OLODATEROL OU CHLORHYDRATE D'OLODATEROL; NAT. REGISTRATION NO/DATE: NL42395 20140522; FIRST REGISTRATION: MT - MA211/00401 20130918 |
| 1562603 | 2014/016 | Ireland | ⤷ Sign Up | PRODUCT NAME: OLODATEROL, OPTICAL ISOMERS THEREOF, MIXTURES OF ISOMERS THEREOF, ACID ADDITION SALTS THEREOF WITH PHARMACOLOGICALLY ACCEPTABLE ACIDS, AS WELL AS SOLVATES AND/OR HYDRATES THEREOF, IN PARTICULAR OLODATEROL AND OLODATEROL HYDROCHLORIDE.; NAT REGISTRATION NO/DATE: PA0775/006/001 20131018; FIRST REGISTRATION NO/DATE: MA211/00401 20130918 |
| 1562603 | 132014902316088 | Italy | ⤷ Sign Up | PRODUCT NAME: OLODATEROLO, SUOI SINGOLI ISOMERI OTTICI, MISCELE DEI SINGOLI ENANTIOMERI O DI RACEMATI, SUOI SALI DI ADDIZIONE CON ACIDI FARMACOLOGICAMENTE ACCETTABILI NONCHE' SUOI SOLVATI E/O IDRATI, IN PARTICOLARE OLODATEROLO E OLODATEROLO CLORIDRATO(STRIVERDI RESPIMAT); AUTHORISATION NUMBER(S) AND DATE(S): MA211/00401, 20130918;042432017-029-031-043, 20140618 |
| 1562603 | 21/2014 | Austria | ⤷ Sign Up | PRODUCT NAME: OLODATEROL, DESSEN SAEUREHAELTIGEN SALZE MIT PHARMAKOLOGISCH VERTRAEGLICHEN SAEUREN, IM BESONDEREN OLODATEROL HYDROCHLORIDE; NAT. REGISTRATION NO/DATE: 135232 20131125; FIRST REGISTRATION: MT MA211/00401 20130918 |
| 1562603 | 513 | Finland | ⤷ Sign Up | |
| 1562603 | CR 2014 00014 | Denmark | ⤷ Sign Up | PRODUCT NAME: OLODATEROL, OPTISKE ISOMERER DERAF, BLANDINGER AF ISOMERER DERAF, SYREADDITIONSSALTE DERAF MED FARMAKOLGISK HARMLOESE SYRER, SAVEL SOM SOLVATER OG/ELLER HYDRATER DERAF, SAERLIGT OLODATEROL OG OLODATEROL HYDROCHLORID; NAT. REG. NO/DATE: 50975 20131014; FIRST REG. NO/DATE: MA 211/00401 20130918 |
| 1562603 | 1490016-1 | Sweden | ⤷ Sign Up | PRODUCT NAME: OLODATEROL, INDIVIDUELLA OPTISKA ISOMERER DAERAV, BLANDNINGAR AV DE SEPARATA ENANTIOMERERNA ELLER RACEMATEN DAERAV, SYRA ADDITIONSSALTER DAERAV MED FARMAKOLOGISKT GODTAGBARA SYROR SAVAEL SOM SOLVATER OCH/ELLER HYDRATER DAERAV, SAERSKILT OLODATEROL OCH OLODATEROLHYDROKLORID; NAT. REG. NO/DATE: MT-NR 48116 20131114; FIRST REG.: MT MA 211/00401 20130918 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


