SIVEXTRO Drug Patent Profile
✉ Email this page to a colleague
When do Sivextro patents expire, and what generic alternatives are available?
Sivextro is a drug marketed by Cubist Pharms Llc and is included in two NDAs. There are seven patents protecting this drug.
This drug has seventy-nine patent family members in thirty-nine countries.
The generic ingredient in SIVEXTRO is tedizolid phosphate. Two suppliers are listed for this compound. Additional details are available on the tedizolid phosphate profile page.
DrugPatentWatch® Generic Entry Outlook for Sivextro
Sivextro was eligible for patent challenges on June 20, 2018.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be December 31, 2030. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for SIVEXTRO?
- What are the global sales for SIVEXTRO?
- What is Average Wholesale Price for SIVEXTRO?
Summary for SIVEXTRO
| International Patents: | 79 |
| US Patents: | 7 |
| Applicants: | 1 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 62 |
| Clinical Trials: | 5 |
| Patent Applications: | 172 |
| Drug Prices: | Drug price information for SIVEXTRO |
| What excipients (inactive ingredients) are in SIVEXTRO? | SIVEXTRO excipients list |
| DailyMed Link: | SIVEXTRO at DailyMed |
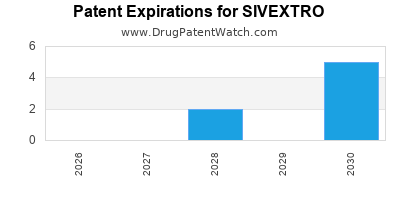

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for SIVEXTRO
Generic Entry Dates for SIVEXTRO*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
POWDER;INTRAVENOUS |
Generic Entry Dates for SIVEXTRO*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for SIVEXTRO
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Assistance Publique - Hôpitaux de Paris | Phase 2 |
| Lundquist Institute for Biomedical Innovation at Harbor-UCLA Medical Center | Phase 2 |
| Los Angeles Biomedical Research Institute | Phase 2 |
Pharmacology for SIVEXTRO
| Drug Class | Oxazolidinone Antibacterial |
| Mechanism of Action | Breast Cancer Resistance Protein Inhibitors |
US Patents and Regulatory Information for SIVEXTRO
SIVEXTRO is protected by seven US patents and one FDA Regulatory Exclusivity.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of SIVEXTRO is ⤷ Get Started Free.
This potential generic entry date is based on patent ⤷ Get Started Free.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
EU/EMA Drug Approvals for SIVEXTRO
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Merck Sharp & Dohme B.V. | Sivextro | tedizolid phosphate | EMEA/H/C/002846Sivextro is indicated for the treatment of acute bacterial skin and skin structure infections (ABSSSI) in adults and adolescents 12 years of age and older. | Authorised | no | no | no | 2015-03-23 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for SIVEXTRO
When does loss-of-exclusivity occur for SIVEXTRO?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
African Regional IP Organization (ARIPO)
Patent: 87
Patent: Crystalline form of (R)-3-(-4(2-(2-methyltetrazol-5-YL)pyridin-5-YL)-3-fluorophenyl)-5-hydroxymethyloxazolidin-2-one dihydrogen phosphate
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 10210627
Patent: Crystalline form of R)-3-(4-(2-(2-methyltetrazol-5-yl)pyridin-5-yl)-3-fluorophenyl)-5-hydroxymethyl oxazolidin-2-one dihydrogen phosphate
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 1008829
Patent: forma cristalina de dihidrogenofosfato de (r)-3-(4-(2-(2-metiltetrazol-5-il) piridin-5-il)-3-fluorofenil)-5-hidroximetil oxazolidin-2-ona
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 51392
Patent: FORME CRISTALLINE DU DIHYDROGENOPHOSPHATE DE (R)-3-(4-(2-(2-METHYLTETRAZOL-5-YL)PYRIDIN-5-YL)-3-FLUOROPHENYL)-5-HYDROXYMETHYL OXAZOLIDIN-2-ONE (CRYSTALLINE FORM OF R)-3-(4-(2-(2-METHYLTETRAZOL-5-YL)PYRIDIN-5-YL)-3-FLUOROPHENYL)-5-HYDROXYMETHYL OXAZOLIDIN-2-ONE DIHYDROGEN PHOSPHATE)
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 11001855
Patent: Partículas cristalinas que comprenden al compuesto fosfato de dihidrógeno de (r)-3-(4-(2-(2-metiltetrazol-5-il)piridin-5-il)-3-fluorofenil)-5-hidroximetil oxazolidin-2-ona; proceso de preparación; mezcla que la comprende; composición farmacéutica; y su uso para tratar una infección bacteriana.
Estimated Expiration: ⤷ Get Started Free
China
Patent: 2439006
Patent: Crystalline form of r)-3-(4-(2-(2-methyltetrazol-5-yl)pyridin-5-yl)-3-fluorophenyl)-5-hydroxymethyl oxazolidin-2-one dihydrogen phosphate
Estimated Expiration: ⤷ Get Started Free
Patent: 7082790
Patent: 种噁唑烷酮化合物的晶型 (Crystalline form of R)-3-(4-(2-(2-methyltetrazol-5-yl)pyridin-5-yl)-3-fluorophenyl)-5-hydroxymethyl oxazolidin-2-one dihydrogen phosphate)
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 20071
Patent: Forma cristalina del fosfato de dihidrogeno r)-3-(4-(2-(2-metiltetrazol-5-il)piridin-5-il)-3-fluorofenil)-5-hidroximetil oxazolidin-2-ona
Estimated Expiration: ⤷ Get Started Free
Costa Rica
Patent: 110464
Patent: FORMA CRISTALINA DEL FOSFATO DE DIHIDROGENO R)-3-(4-(2-(2-METILTETRAZOL-5-IL)PIRIDIN-5-IL)-3-FLUOROFENIL)-5- HIDROXIMETIL OXAZOLIDIN-2-ONA
Estimated Expiration: ⤷ Get Started Free
Cuba
Patent: 089
Patent: FORMA CRISTALINA DEL FOSFATO DE DIHIDRÓGENO R)-3-(4-(2-(2-METILTRETAZOL-5-IL)-3-FLUOROFENIL)-5-HIDROXIMETIL OXAZOLIDIN-2-ONA
Estimated Expiration: ⤷ Get Started Free
Patent: 110155
Patent: FORMA CRISTALINA DEL FOSFATO DE DIHIDRÓGENO R)-3-(4-(2-(2-METILTRETAZOL-5-IL)-3-FLUOROFENIL)-5-HIDROXIMETIL OXAZOLIDIN-2-ONA
Estimated Expiration: ⤷ Get Started Free
Dominican Republic
Patent: 011000251
Patent: FORMA CRISTALINA DEL FOSFATO DE DIHIDROGENO R)-3-(4-(2-(2-METILTETRAZOL-5-IL) PIRIDIN-5-IL)-3-FLUOROFENIL)-5-HIDROXIMETIL OXAZOLIDIN-2-ONA
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 11011285
Patent: FORMA CRISTALINA DEL FOSFATO DE DIHIDROGENO R)-3-(4-(2-(2-METILTETRAZOL-5-IL)PIRIDIN-5-IL)-3-FLUOROFENIL)-5-HIDROXIMETIL OXAZOLIDIN-2-ONA
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 93808
Patent: FORME CRISTALLINE DU DIHYDROGÉNOPHOSPHATE DE (R)-3-(4-(2-(2-MÉTHYLTÉTRAZOL-5-YL)PYRIDIN-5-YL)-3-FLUOROPHÉNYL)-5-HYDROXYMÉTHYL OXAZOLIDIN-2-ONE (CRYSTALLINE FORM OF R)-3-(4-(2-(2-METHYLTETRAZOL-5-YL)PYRIDIN-5-YL)-3-FLUOROPHENYL)-5-HYDROXYMETHYL OXAZOLIDIN-2-ONE DIHYDROGEN PHOSPHATE)
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 4401
Patent: צורה גבישית של (r)-3-(4-(2-(2-מתילטטראזול-5-יל)פירידין-5-יל)-3-פלואורופניל)-5-הידרוקסימתיל אוקסאזולידין-2-און דיהידרוגן פוספאט (Crystalline form of (r)-3-(4-(2-(2-methyltetrazol-5-yl)pyridin-5-yl)-3-fluorophenyl)-5-hydroxymethyl oxazolidin-2-one dihydrogen phosphate)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 84705
Estimated Expiration: ⤷ Get Started Free
Patent: 12516894
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 6354
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 4300
Estimated Expiration: ⤷ Get Started Free
Patent: 11008093
Estimated Expiration: ⤷ Get Started Free
Patent: 20011773
Estimated Expiration: ⤷ Get Started Free
Morocco
Patent: 092
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 4408
Estimated Expiration: ⤷ Get Started Free
Patent: 0458
Estimated Expiration: ⤷ Get Started Free
Patent: 2289
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 120585
Estimated Expiration: ⤷ Get Started Free
Philippines
Patent: 014500092
Estimated Expiration: ⤷ Get Started Free
Russian Federation
Patent: 55928
Estimated Expiration: ⤷ Get Started Free
Patent: 11136537
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 3497
Estimated Expiration: ⤷ Get Started Free
Patent: 201500207Q
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1106412
Estimated Expiration: ⤷ Get Started Free
Patent: 1306536
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1739923
Estimated Expiration: ⤷ Get Started Free
Patent: 1918678
Estimated Expiration: ⤷ Get Started Free
Patent: 110120311
Patent: CRYSTALLINE FORM OF R)-3-(4-(2-(2-METHYLTETRAZOL-5-YL)PYRIDIN-5-YL)-3-FLUOROPHENYL)-5-HYDROXYMETHYL OXAZOLIDIN-2-ONE DIHYDROGEN PHOSPHATE
Estimated Expiration: ⤷ Get Started Free
Patent: 170040371
Patent: R)-3--5-히드록시메틸 옥사졸리딘-2-온 디히드로겐 포스페이트의 결정형 (-3-4-2-2--5--5--3--5- -2- Crystalline form of R-3-4-2-2-methyltetrazol-5-ylpyridin-5-yl-3-fluorophenyl-5-hydroxymethyl oxazolidin-2-one dihydrogen phosphate)
Estimated Expiration: ⤷ Get Started Free
Patent: 170135984
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 34724
Estimated Expiration: ⤷ Get Started Free
Tunisia
Patent: 11000381
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 4068
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering SIVEXTRO around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Japan | 5584705 | ⤷ Get Started Free | |
| China | 107082790 | 种噁唑烷酮化合物的晶型 (Crystalline form of R)-3-(4-(2-(2-methyltetrazol-5-yl)pyridin-5-yl)-3-fluorophenyl)-5-hydroxymethyl oxazolidin-2-one dihydrogen phosphate) | ⤷ Get Started Free |
| Japan | 2012516894 | ⤷ Get Started Free | |
| World Intellectual Property Organization (WIPO) | 2010091131 | ⤷ Get Started Free | |
| Israel | 214401 | ⤷ Get Started Free | |
| Hong Kong | 1155747 | OXAZOLIDINONE DERIVATIVES | ⤷ Get Started Free |
| Japan | 5584705 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for SIVEXTRO
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1699784 | 675 | Finland | ⤷ Get Started Free | |
| 1699784 | SPC/GB15/056 | United Kingdom | ⤷ Get Started Free | PRODUCT NAME: TEDIZOLID, OPTIONALLY IN THE FORM OF AN ESTER, IN PARTICULAR A PHOSPHATE, OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF; REGISTERED: UK EU/1/15/991 20150325 |
| 1699784 | 132016000024232 | Italy | ⤷ Get Started Free | PRODUCT NAME: TEDIZOLID, FACOLTATIVAMENTE IN FORMA DI ESTERE, IN PARTICOLARE UN FOSFATO, O UN SUO SALE FARMACEUTICAMENTE ACCETTABILE(SIVEXTRO); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/15/991/001, 20150325 |
| 1699784 | 243 50021-2015 | Slovakia | ⤷ Get Started Free | PRODUCT NAME: TEDIZOLIDFOSFAT; REGISTRATION NO/DATE: EU/1/15/991/001 - EU/1/15/991/003 20150325 |
| 1699784 | 122015000078 | Germany | ⤷ Get Started Free | PRODUCT NAME: TEDIZOLID, GEGEBENENFALLS IN FORM EINES ESTERS, INSBESONDERE EINES PHOSPHATS, ODER EIN PHARMAZEUTISCH VERTRAEGLICHES SALZ DAVON; REGISTRATION NO/DATE: EU/1/15/991/001-003 20150323 |
| 1699784 | CA 2015 00048 | Denmark | ⤷ Get Started Free | PRODUCT NAME: TEDIZOLID, OPTIONALLY IN THE FORM OF AN ESTER, IN PARTICULAR A PHOSPHATE, OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF, INCLUDING TEDIZOLID PHOSPHATE; REG. NO/DATE: EU/1/15/991 20150325 |
| 1699784 | PA2015032,C1699784 | Lithuania | ⤷ Get Started Free | PRODUCT NAME: TEDIZOLIDAS, PASIRINKTINAI ESTERIO PAVIDALU, YPAC FOSFATO ARBA JO FARMACINIU POZIURIU PRIIMTINA DRUSKA; REGISTRATION NO/DATE: EU/1/15/991 20150323 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for SIVEXTRO (Xeflim Functional Sodium)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
