SIMBRINZA Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Simbrinza, and when can generic versions of Simbrinza launch?
Simbrinza is a drug marketed by Alcon Labs Inc and is included in one NDA. There are two patents protecting this drug and one Paragraph IV challenge.
This drug has fifty-one patent family members in twenty-six countries.
The generic ingredient in SIMBRINZA is brimonidine tartrate; brinzolamide. There are eleven drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the brimonidine tartrate; brinzolamide profile page.
DrugPatentWatch® Generic Entry Outlook for Simbrinza
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be June 17, 2030. This may change due to patent challenges or generic licensing.
There has been one patent litigation case involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for SIMBRINZA?
- What are the global sales for SIMBRINZA?
- What is Average Wholesale Price for SIMBRINZA?
Summary for SIMBRINZA
| International Patents: | 51 |
| US Patents: | 2 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 2 |
| Clinical Trials: | 10 |
| Patent Applications: | 53 |
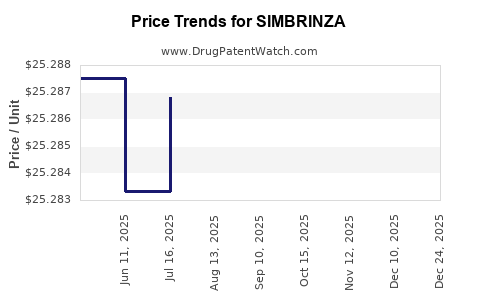
| Drug Prices: | Drug price information for SIMBRINZA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for SIMBRINZA |
| What excipients (inactive ingredients) are in SIMBRINZA? | SIMBRINZA excipients list |
| DailyMed Link: | SIMBRINZA at DailyMed |
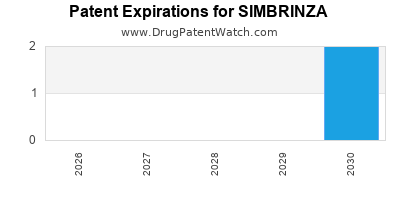
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for SIMBRINZA
Generic Entry Date for SIMBRINZA*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
SUSPENSION/DROPS;OPHTHALMIC |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for SIMBRINZA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Prairie Eye Center | PHASE4 |
| Sengi | PHASE4 |
| Perrigo Company | Phase 3 |
Pharmacology for SIMBRINZA
| Drug Class | Carbonic Anhydrase Inhibitor alpha-Adrenergic Agonist |
| Mechanism of Action | Adrenergic alpha-Agonists Carbonic Anhydrase Inhibitors |
Paragraph IV (Patent) Challenges for SIMBRINZA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| SIMBRINZA | Ophthalmic Suspension | brimonidine tartrate; brinzolamide | 1%/0.2% | 204251 | 1 | 2022-08-01 |
US Patents and Regulatory Information for SIMBRINZA
SIMBRINZA is protected by two US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of SIMBRINZA is ⤷ Get Started Free.
This potential generic entry date is based on patent 9,421,265.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alcon Labs Inc | SIMBRINZA | brimonidine tartrate; brinzolamide | SUSPENSION/DROPS;OPHTHALMIC | 204251-001 | Apr 19, 2013 | RX | Yes | Yes | 9,044,484 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Alcon Labs Inc | SIMBRINZA | brimonidine tartrate; brinzolamide | SUSPENSION/DROPS;OPHTHALMIC | 204251-001 | Apr 19, 2013 | RX | Yes | Yes | 9,421,265 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for SIMBRINZA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Alcon Labs Inc | SIMBRINZA | brimonidine tartrate; brinzolamide | SUSPENSION/DROPS;OPHTHALMIC | 204251-001 | Apr 19, 2013 | 6,316,441 | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for SIMBRINZA
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Novartis Europharm Limited | Simbrinza | brinzolamide, brimonidine tartrate | EMEA/H/C/003698Decrease of elevated intraocular pressure (IOP) in adult patients with open-angle glaucoma or ocular hypertension for whom monotherapy provides insufficient IOP reduction. | Authorised | no | no | no | 2014-07-18 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for SIMBRINZA
When does loss-of-exclusivity occur for SIMBRINZA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 7126
Patent: COMPOSICIONES FARMACEUTICAS ACUOSAS QUE CONTIENEN COMPLEJOS DE BORATO-POLIOL
Estimated Expiration: ⤷ Get Started Free
Patent: 2017
Patent: COMPOSICIONES FARMACÉUTICAS ACUOSAS QUE CONTIENEN COMPLEJOS DE BORATO-POLIOL
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 10262898
Patent: Aqueous pharmaceutical compositions containing borate-polyol complexes
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 1015996
Patent: composições farmacêuticas aquosas contendo complexos de borato-poliol
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 63778
Patent: COMPOSITIONS PHARMACEUTIQUES AQUEUSES CONTENANT DES COMPLEXES BORATE-POLYOLS (AQUEOUS PHARMACEUTICAL COMPOSITIONS CONTAINING BORATE-POLYOL COMPLEXES)
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 10000634
Patent: Composicion oftalmica multidosis que comprende un primer poliol seleccionado de manitol y/o sorbitol, un segundo poliol seleccionado de propilenglicol y/o glicerina, borato en cantidad menor a 0,5% en p/v, cloruro de benzalconio en concentracion de 0.0007%-0,0035% p/v y agua y uso para el tratamiento de la presion intraocular elevada.
Estimated Expiration: ⤷ Get Started Free
China
Patent: 2802604
Patent: Aqueous pharmaceutical compositions containing borate-polyol complexes
Estimated Expiration: ⤷ Get Started Free
Patent: 4707145
Patent: Aqueous pharmaceutical compositions containing borate-polyol complexes
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0160953
Estimated Expiration: ⤷ Get Started Free
Patent: 0200979
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 17835
Estimated Expiration: ⤷ Get Started Free
Patent: 23120
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 42790
Estimated Expiration: ⤷ Get Started Free
Patent: 22035
Estimated Expiration: ⤷ Get Started Free
Patent: 45164
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 42790
Patent: COMPOSITIONS PHARMACEUTIQUES AQUEUSES CONTENANT DES COMPLEXES BORATE-POLYOLS (AQUEOUS PHARMACEUTICAL COMPOSITIONS CONTAINING BORATE-POLYOL COMPLEXES)
Estimated Expiration: ⤷ Get Started Free
Patent: 22035
Patent: Compositions pharmaceutiques aqueuses contenant des complexes de borate-polyol (Aqueous pharmaceutical compositions containing borate-polyol complexes)
Estimated Expiration: ⤷ Get Started Free
Patent: 45164
Patent: COMPOSITIONS PHARMACEUTIQUES AQUEUSES CONTENANT DES COMPLEXES DE BORATE-POLYOL (AQUEOUS PHARMACEUTICAL COMPOSITIONS CONTAINING BORATE-POLYOL COMPLEXES)
Estimated Expiration: ⤷ Get Started Free
Patent: 37634
Patent: COMPOSITIONS PHARMACEUTIQUES AQUEUSES CONTENANT DES COMPLEXES DE BORATE-POLYOL (AQUEOUS PHARMACEUTICAL COMPOSITIONS CONTAINING BORATE-POLYOL COMPLEXES)
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 63521
Patent: 含有硼酸鹽多元醇混合物的水溶性藥物組合物 (AQUEOUS PHARMACEUTICAL COMPOSITIONS CONTAINING BORATE-POLYOL COMPLEXES)
Estimated Expiration: ⤷ Get Started Free
Patent: 94007
Patent: 含有硼酸鹽多元醇混合物的水溶性藥物組合物 (AQUEOUS PHARMACEUTICAL COMPOSITIONS CONTAINING BORATE-POLYOL COMPLEXES)
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 30435
Estimated Expiration: ⤷ Get Started Free
Patent: 49477
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 17956
Estimated Expiration: ⤷ Get Started Free
Patent: 12530712
Estimated Expiration: ⤷ Get Started Free
Patent: 14198729
Patent: ボレート−ポリオール複合体を含む水性薬学的組成物 (AQUEOUS PHARMACEUTICAL COMPOSITIONS CONTAINING BORATE-POLYOL COMPLEXES)
Estimated Expiration: ⤷ Get Started Free
Patent: 16183198
Patent: ボレート−ポリオール複合体を含む水性薬学的組成物 (AQUEOUS PHARMACEUTICAL COMPOSITIONS CONTAINING BORATE-POLYOL COMPLEXES)
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 45164
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 11013107
Patent: COMPOSICIONES FARMACEUTICAS ACUOSAS QUE CONTIENEN COMPLEJOS DE BORATO - POLIOL. (AQUEOUS PHARMACEUTICAL COMPOSITIONS CONTAINING BORATE-POLYOL COMPLEXES.)
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 42790
Estimated Expiration: ⤷ Get Started Free
Patent: 22035
Estimated Expiration: ⤷ Get Started Free
Patent: 45164
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 42790
Estimated Expiration: ⤷ Get Started Free
Patent: 22035
Estimated Expiration: ⤷ Get Started Free
Patent: 45164
Estimated Expiration: ⤷ Get Started Free
Russian Federation
Patent: 63125
Patent: ВОДНЫЕ ФАРМАЦЕВТИЧЕСКИЕ КОМПОЗИЦИИ, СОДЕРЖАЩИЕ КОМПЛЕКСЫ БОРАТПОЛИОЛ (AQUEOUS PHARMACEUTICAL COMPOSITIONS, CONTAINING BORATE-POLYOL COMPLEXES)
Estimated Expiration: ⤷ Get Started Free
Patent: 12101782
Patent: ВОДНЫЕ ФАРМАЦЕВТИЧЕСКИЕ КОМПОЗИЦИИ, СОДЕРЖАЩИЕ КОМПЛЕКСЫ БОРАТПОЛИОЛ
Estimated Expiration: ⤷ Get Started Free
San Marino
Patent: 01600249
Patent: COMPOSIZIONI FARMACEUTICHE ACQUOSE CONTENENTI COMPLESSI BORATO- POLIOLO
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 42790
Estimated Expiration: ⤷ Get Started Free
Patent: 22035
Estimated Expiration: ⤷ Get Started Free
Patent: 45164
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1108384
Patent: AQUEOUS PHARMACEUTICAL COMPOSITIONS CONTAINING BORATE-POLYOL COMPLEX
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1738502
Estimated Expiration: ⤷ Get Started Free
Patent: 120028390
Patent: AQUEOUS PHARMACEUTICAL COMPOSITIONS CONTAINING BORATE-POLYOL COMPLEXES
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 61617
Estimated Expiration: ⤷ Get Started Free
Patent: 84858
Estimated Expiration: ⤷ Get Started Free
Patent: 03648
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 89997
Estimated Expiration: ⤷ Get Started Free
Patent: 1100103
Patent: Aqueous pharmaceutical compositions containing borate-polyol complexes
Estimated Expiration: ⤷ Get Started Free
Uruguay
Patent: 709
Patent: COMPOSICIONES FARMACÉUTICAS ACUOSAS QUE CONTIENEN COMPLEJOS BORATO-POLIOL
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering SIMBRINZA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| European Patent Office | 2442790 | COMPOSITIONS PHARMACEUTIQUES AQUEUSES CONTENANT DES COMPLEXES BORATE-POLYOLS (AQUEOUS PHARMACEUTICAL COMPOSITIONS CONTAINING BORATE-POLYOL COMPLEXES) | ⤷ Get Started Free |
| Denmark | 3045164 | ⤷ Get Started Free | |
| Mexico | 2011013107 | COMPOSICIONES FARMACEUTICAS ACUOSAS QUE CONTIENEN COMPLEJOS DE BORATO - POLIOL. (AQUEOUS PHARMACEUTICAL COMPOSITIONS CONTAINING BORATE-POLYOL COMPLEXES.) | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for SIMBRINZA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1631293 | 300683 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: BRIMONIDINE EN FARMACEUTISCH AANVAARDBARE ZOUTEN DAARVAN VOOR TOEPASSING ALS GENEESMIDDEL VOOR DE BEHANDELING VAN DOOR ROSACEA GEINDUCEERDE ROODHEID; REGISTRATION NO/DATE: EU/1/13/904 20140225 |
| 1631293 | C300683 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: BRIMONIDINE EN FARMACEUTISCH AANVAARDBARE ZOUTEN DAARVAN VOOR TOEPASSING ALS GENEESMIDDEL VOOR DE BEHANDELING VAN DOOR ROSACEA GEINDUCEERDE ROODHEID; REGISTRATION NO/DATE: EU/1/13/904 20140225 |
| 1631293 | 14C0056 | France | ⤷ Get Started Free | PRODUCT NAME: BRIMONIDINE OU L'UN DE SES SELS PHARMACEUTIQUEMENT ACCEPTABLES; REGISTRATION NO/DATE: EU/1/13/904 20140225 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for Simbrinza
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
