REYVOW Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Reyvow, and what generic alternatives are available?
Reyvow is a drug marketed by Eli Lilly And Co and is included in one NDA. There are four patents protecting this drug and one Paragraph IV challenge.
This drug has one hundred and eighty-seven patent family members in forty-seven countries.
The generic ingredient in REYVOW is lasmiditan succinate. One supplier is listed for this compound. Additional details are available on the lasmiditan succinate profile page.
DrugPatentWatch® Generic Entry Outlook for Reyvow
Reyvow was eligible for patent challenges on January 31, 2024.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be December 5, 2037. This may change due to patent challenges or generic licensing.
There is one Paragraph IV patent challenge for this drug. This may lead to patent invalidation or a license for generic production.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for REYVOW?
- What are the global sales for REYVOW?
- What is Average Wholesale Price for REYVOW?
Summary for REYVOW
| International Patents: | 187 |
| US Patents: | 4 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 84 |
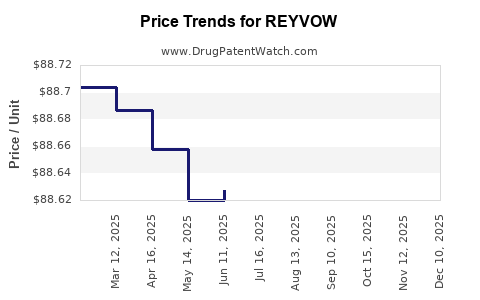
| Drug Prices: | Drug price information for REYVOW |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for REYVOW |
| What excipients (inactive ingredients) are in REYVOW? | REYVOW excipients list |
| DailyMed Link: | REYVOW at DailyMed |
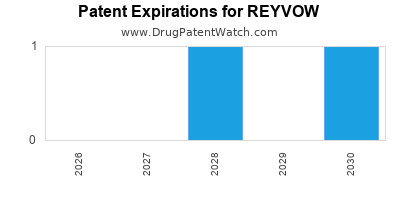
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for REYVOW
Generic Entry Date for REYVOW*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Paragraph IV (Patent) Challenges for REYVOW
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| REYVOW | Tablets | lasmiditan succinate | 50 mg and 100 mg | 211280 | 1 | 2024-01-31 |
US Patents and Regulatory Information for REYVOW
REYVOW is protected by four US patents and one FDA Regulatory Exclusivity.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of REYVOW is ⤷ Get Started Free.
This potential generic entry date is based on patent ⤷ Get Started Free.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Expired US Patents for REYVOW
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Eli Lilly And Co | REYVOW | lasmiditan succinate | TABLET;ORAL | 211280-001 | Jan 31, 2020 | ⤷ Get Started Free | ⤷ Get Started Free |
| Eli Lilly And Co | REYVOW | lasmiditan succinate | TABLET;ORAL | 211280-003 | Dec 18, 2020 | ⤷ Get Started Free | ⤷ Get Started Free |
| Eli Lilly And Co | REYVOW | lasmiditan succinate | TABLET;ORAL | 211280-002 | Jan 31, 2020 | ⤷ Get Started Free | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for REYVOW
When does loss-of-exclusivity occur for REYVOW?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 17373784
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2019010934
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 43772
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 19001426
Estimated Expiration: ⤷ Get Started Free
China
Patent: 0291079
Estimated Expiration: ⤷ Get Started Free
Patent: 5385893
Estimated Expiration: ⤷ Get Started Free
Patent: 5385894
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 19005290
Estimated Expiration: ⤷ Get Started Free
Costa Rica
Patent: 190251
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0211557
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 24540
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 51617
Estimated Expiration: ⤷ Get Started Free
Dominican Republic
Patent: 019000139
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 19040190
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 1991112
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 51617
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 56820
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 6598
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 61240
Estimated Expiration: ⤷ Get Started Free
Patent: 20500936
Estimated Expiration: ⤷ Get Started Free
Patent: 22000451
Estimated Expiration: ⤷ Get Started Free
Patent: 23123678
Estimated Expiration: ⤷ Get Started Free
Patent: 24178323
Estimated Expiration: ⤷ Get Started Free
Jordan
Patent: 0190129
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 51617
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 6855
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 8091
Estimated Expiration: ⤷ Get Started Free
Patent: 2547
Estimated Expiration: ⤷ Get Started Free
Patent: 19006520
Estimated Expiration: ⤷ Get Started Free
Patent: 21014139
Estimated Expiration: ⤷ Get Started Free
Moldova, Republic of
Patent: 51617
Estimated Expiration: ⤷ Get Started Free
Morocco
Patent: 920
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 2906
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 191134
Estimated Expiration: ⤷ Get Started Free
Patent: 241297
Estimated Expiration: ⤷ Get Started Free
Philippines
Patent: 019501252
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 51617
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 51617
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 415
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 51617
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1903449
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 190075130
Estimated Expiration: ⤷ Get Started Free
Patent: 210102497
Estimated Expiration: ⤷ Get Started Free
Patent: 230008257
Estimated Expiration: ⤷ Get Started Free
Patent: 250070124
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 89476
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 71290
Estimated Expiration: ⤷ Get Started Free
Patent: 1833097
Estimated Expiration: ⤷ Get Started Free
Tunisia
Patent: 19000174
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 4433
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering REYVOW around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Mexico | 2021014139 | ⤷ Get Started Free | |
| South Africa | 202303699 | ⤷ Get Started Free | |
| Lithuania | 3551617 | ⤷ Get Started Free | |
| China | 107412230 | ⤷ Get Started Free | |
| Croatia | P20040883 | PYRIDINOYLPIPERIDINES AS 5-HT1F AGONISTS | ⤷ Get Started Free |
| Japan | 7566996 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for REYVOW
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2413933 | 2390001-2 | Sweden | ⤷ Get Started Free | PRODUCT NAME: LASMIDITAN OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF; REG. NO/DATE: EU/1/21/1587 20220819 |
| 2413933 | CA 2023 00001 | Denmark | ⤷ Get Started Free | PRODUCT NAME: LASMIDITAN ELLER ET FARMACEUTISK ACCEPTABELT SALT DERAF; REG. NO/DATE: EU/1/21/1587 20220819 |
| 2413933 | 2023C/503 | Belgium | ⤷ Get Started Free | PRODUCT NAME: LASMIDITAN; AUTHORISATION NUMBER AND DATE: EU/1/21/1587 20220819 |
| 2413933 | PA2023502,C2413933 | Lithuania | ⤷ Get Started Free | PRODUCT NAME: LASMIDITANAS ARBA JO FARMACINIU POZIURIU PRIIMTINA DRUSKA; REGISTRATION NO/DATE: EU/1/21/1587 20220817 |
| 2413933 | SPC/GB23/001 | United Kingdom | ⤷ Get Started Free | PRODUCT NAME: LASMIDITAN; REGISTERED: UK EU/1/21/1587(FOR NI) 20220819 |
| 2413933 | C202330007 | Spain | ⤷ Get Started Free | PRODUCT NAME: LASMIDITAN O UNA SAL FARMACEUTICAMENTE ACEPTABLE DEL MISMO; NATIONAL AUTHORISATION NUMBER: EU/1/21/1587; DATE OF AUTHORISATION: 20220817; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/21/1587; DATE OF FIRST AUTHORISATION IN EEA: 20220817 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for REYVOW (Lasmiditan)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
